2627
The value of MRI in radiation therapy1Radiation Oncology, Washington University in St Louis, St Louis, MO, United States, 2Radiation Oncology, Barnes-Jewish Hospital, St Louis, MO, United States, 3MRI, Philips Healthcare, Cleveland, OH, United States
Synopsis
During the last decade, the role of MRI in radiation therapy (RT) grew dramatically. The soft-tissue benefits from MRI simulations complement the geometric accuracy and photon attenuation maps from computed tomography in RT treatment planning. MR for calculating attenuation (MRCAT) is being used for MRI-only treatment planning. The clinical utilization of hybrid MRI-guided radiation therapy (MR-IGRT) systems began in January 2014. MR-IGRT enables real-time tracking of tumors and highly conformal treatments that enable improved patient outcomes. Hence, the value of MRI in RT is rapidly rising. Examples of MRI's role in, and value to, RT are presented.
Purpose
The value of MRI in radiation therapy is driving one of MRI's fastest growing applications. MRI has three main roles in radiation therapy (RT): 1) Disease diagnosis and treatment response typically managed through radiology departments; 2) Treatment planning using MRI simulations for delineation of tumor and organs at risk (OARs) and optionally MR for calculating attenuation (MRCAT); and 3) MR image-guided radiation therapy (MR-IGRT).1,2
Despite the lack of insurance reimbursement, large radiation oncology departments are purchasing MRI systems equipped with external laser positioning systems (ELPS), flat table inserts, and RT-optimized pulse sequences to perform MRI simulations, and staffing the systems with MR technologists and physicists. The increasing role of MRI in RT is motivated by its superior soft tissue contrast in treatment planning and therapy, and the associated benefits that improved organ delineation may have on the outcomes of radiotherapy.
In this study, we provide an overview of the role and value of MRI in RT treatment planning and treatment, and discuss the future opportunities and needs for the field.
Methods
Simulation: At our institution, 20% of patients receive both MRI and CT simulations (Fig. 1). The CT and MRI images are fused and contours of the tumor and organs at risk are manually drawn as part of treatment planning. MRI simulation is performed on a 1.5 T Philips Ingenia (Cleveland, OH, V5.3) equipped with RT features including an ELPS and flat patient table. MRCAT, metal artifact reduction, and motion correction (MultiVane) sequences augment the conventional MRI acquisitions.
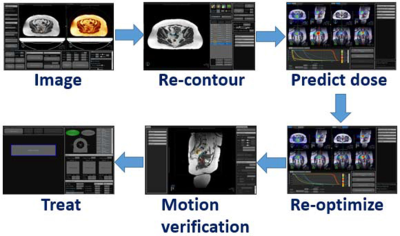
MR-IGRT: At least ten percent of our patients are treated using a ViewRay 0.35 T MRIdian with either three 60Co heads or a Linac. Key benefits of MR-IGRT are the capabilities to: 1) Visualize the tumor (Fig. 2) and track its motion versus cone-beam CT image guided radiotherapy (CBCT-IGRT); 2) Gate the dose delivery based on the tumor's position; 3) Adapt the RT while the patient lays in the system (Fig. 3); 4) and administer highly conformal radiation doses (e.g., hypofractionated or stereotactic RT) while sparing healthy tissue.3-6
Results
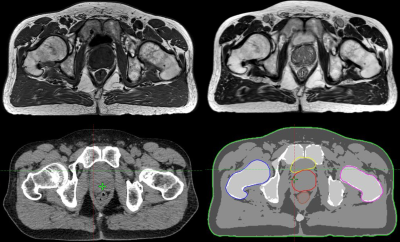
Simulation: Fig. 4 shows the benefits of MRI contrast versus CT in a prostate cancer patient. MRCAT pseudo CTs and autocontours were generated from axial 3D T1-weighted (T1W) mDixon and sagittal 3D T2-weighted (T2W) acquisitions.
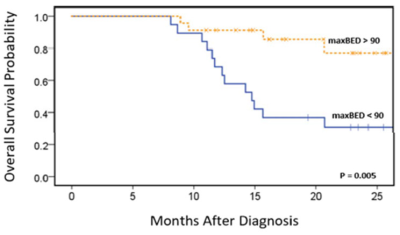
MR-IGRT: In a multicenter trial, high dose RT provided remarkable improvements in overall survival and local control for inoperable pancreatic cancer (Fig. 5). The high doses were enabled by online, on-couch adaptive MR-IGRT and MRI's superior ability to identify and track the target. The early success of this study has led to a Phase II clinical trial.
Discussion
We have shown just two examples of the benefits of MRI in RT. During the last decade, MRI simulation and MR-IGRT became standard practices at large medical institutions covering a large range of cancer applications. The global IGRT market is expected to grow at an annual rate of 18.3%.7 We anticipate 10-20% of IGRT will be performed using MR-IGRT by the end of the next decade. There will be a tremendous need for research, development, and resources (e.g., MR physicists) to support this young field and great opportunities for ISMRM to play a major role. We do not expect the value of CT simulation and CBCT-IGRT to diminish over the same period. Instead, further synergy between the modalities will drive new RT capabilities resulting in improved, highly conformal therapies resulting in reduced dose to healthy tissue.8
The considerations for a MRI in RT differ from diagnostic MRI. The priorities in RT are tumor and OAR delineation with high spatial fidelity. Most of our patients have metal in their bodies that can produce susceptibility artifacts, image distortion, and RF heating. Although 3 T MRI offers increased signal-to-noise ratio over 1.5 T, magnetic susceptibility and inhomogeneity, and spatial distortion increase with field strength.9 Specific absorption rate (SAR) is four times higher at 3 T versus 1.5 T. Dynamic shimming, post-processing, and metal artifact reduction methods can mitigate the effects of metal implants and susceptibility.
Improvements in MRCAT and auto-contouring and their expansion to other anatomies are also needed to minimize dosimetry time and resources. Contouring is largely a manual process. MRCAT in radiology was focused on photon attenuation for PET/MRI. MRCAT in RT will address MRI-only treatment planning for high energy photons, protons and other nuclei (e.g., carbon), and electrons.
Conclusion
The value of MRI in radiation therapy is currently high and is expected to rise dramatically if the current trend of adoption of MR-IGRT and MRI simulation systems continues.Acknowledgements
No acknowledgement found.References
1. Wooten HO, Rodriguez V, Green O, Kashani R, Santanam L, Tanderup K, Mutic S, Li HH. Benchmark IMRT evaluation of a Co-60 MRI-guided radiation therapy system. Radiother Oncol 2015;114(3):402-405.
2. Arivarasan I, Anuradha C, Subramanian S, Anantharaman A, Ramasubramanian V. Magnetic resonance image guidance in external beam radiation therapy planning and delivery. Jpn J Radiol 2017;35(8):417-426.
3. Rudra S, Jiang N, Rosenberg SA, Olsen JR, Parikh PJ, Bassetti MF, Lee P. High dose adaptive MRI guided radiation therapy improves overall survival of inoperable pancreatic cancer. International Journal of Radiation Oncology Biology Physics 2017;99(2 Supplement):E184.
4. Green OL, Gach HM, Mutic S, Cuculich PS, Robinson CG. MRI-directed EP-guided noninvasive cardiac radioablation (ENCORE) for treatment of ventricular tachycardia (VT). International Journal of Radiation Oncology Biology Physics 2017;99(2 Supplement):S123-S124.
5. Kupelian P, Sonke JJ. Magnetic resonance-guided adaptive radiotherapy: a solution to the future. Semin Radiat Oncol 2014;24(3):227-232.
6. Lamb J, Cao M, Kishan A, Agazaryan N, Thomas DH, Shaverdian N, Yang Y, Ray S, Low DA, Raldow A, Steinberg ML, Lee P. Online Adaptive Radiation Therapy: Implementation of a New Process of Care. Cureus 2017;9(8):e1618.
7. MRFR. Image guided radiotherapy market research report- Global forecast till 2023, 2017. Report MRFR/MED/2923-HCRR, 80 p.
8. James AP, Dasarathy BV. Medical image fusion: A survey of the state of the art. Information Fusion 2014;19:4-19.
9. Walker A, Liney G, Metcalfe P, Holloway L. MRI distortion: considerations for MRI based radiotherapy treatment planning. Australas Phys Eng Sci Med 2014;37(1):103-113.
Figures