2625
Estimating Liver Function by Gadoxetate Enhanced MRI: Comparison of Pharmacokinetic Models in a Clinical Setting1Department of Radiation Physics, and Department of Medical and Health Sciences and Center for Medical Image Science and Visualization (CMIV), Linköping Univeristy, Linköping, Sweden, 2Department of Biomedical Engineering, Linköping University, Linköping, Sweden
Synopsis
The hepatic uptake rate of Gadoxetate is a possible biomarker for liver function and several different pharmacokinetic models have been developed. However, no one has ever compared these models using the same data. We compared three different models using imaging data with low temporal, but high spatial resolution. We showed that two of the models estimates almost the same values of the hepatic uptake rate. The fact that two different pharmacokinetic models can produce the same parameter values validates the entire pharmacokinetic modelling approach, indicating that it is not just a model-specific parameter being estimated, but the actual transport rate.
Introduction
Pharmacokinetic parameters, from modeling, of Gadoxetate enhanced MRI, have been proposed as imaging biomarkers for liver function. Several different imaging- and modeling approaches have been developed1-4. Some have used perfusion imaging, with a high temporal resolution1-2, others were based on much lower temporal resolution. We have used the same kind of high-resolution images that are typically acquired in conventional diagnostic Gadoxetate-enhanced protocols3. A clear advantage of this approach is that the liver function measurement can easily be incorporated into conventional clinical practice. Other methods have only been tested in rats4.
To date, none of the pharmacokinetics models has ever been compared using the same data, generated in a clinical setting. Hence, the purpose of this works was to compare the models using data, generated using standard clinical imaging sequences.
Methods
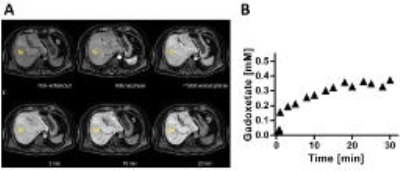
Thirty-five patients with suspected chronic liver disease were included. The patients were imaged using a two-point-Dixon sequence (TR=6.5 ms, TE=2.3/4.6 ms, FA=13°, matrix=168x168, FOV=261x200x342 mm3) both before and up to 30 min after injection of EOB (Fig 1A). The images were reconstructed into water images and regions of interest (ROIs) were placed in the liver (n=7), in the spleen (n=3), in the aorta, and in the portal vein. The average signal intensities in the ROIs were then converted into Gadoxetate concentration5 (Fig 1B).
Three pharmacokinetic models were tested. The first model was based on perfusion2 (Fig 2A) and uses the Gadoxetate concentration in the aorta and portal vein as input functions. The model estimates perfusion parameters as well as the hepatic uptake (ki) and efflux to the bile (kef). The second model was developed using data from rats4 (Fig 2B). Gadoxetate concentrations in the spleen were used as input data to determine the extracellular concentration in the liver. A single ordinary differential equation (ODE) was then used to simulate uptake and efflux of Gadoxetate. The model has only two parameters ki and kef. The third model does not require any input3 (Fig 2C). Instead, it simulates the whole‑body distribution of Gadoxetate using three coupled ODEs. The model have four different parameters: ki, kef, the Gadoxetate backflux to the blood (kback), and a diffusion rate from the blood to the whole‑body extracellular‑extravascular space (kdiff). All three models were fitted to the same datasets using least squares fitting and the pharmacokinetic parameters common in all three models were then compared.
Results
Fig 3 shows the correlation between the hepatic uptake rates, ki, from the different models. Correlation between the perfusion and rat models (Fig 3A; R=0.48), as well as between the perfusion and whole‑body (Fig 3B; R=0.61) were lower than the correlation between the rat and whole‑body models (Fig 3C; R=0.95). Fig 3C also shows that the points in the correlation plot are located almost exactly on the unity line. This means that not only is there a good correlation between the ki estimates, the rat and whole‑body models estimate the same value of the pharmacokinetic parameter.
Fig 4 depicts the correlation between ki estimated using data up to 10
minutes after Gadoxetate injection and ki estimated using data up to
30 minutes after Gadoxetate injection for all three models. As can be seen in
the figure, there is a very good
correlation, indicating that 10 minutes of data is sufficient to estimate ki.
Discussion
This is the first time that pharmacokinetic models for Gadoxetate uptake are compared using data generated from standard clinical sequences. We showed that the models developed by Ulloa4 and Forsgren3 resulted in the same value for the hepatic uptake rate, which is the pharmacokinetic parameter that is most widely suggested as a biomarker for liver function. The fact that two very different pharmacokinetic models provided very similar results validates the entire pharmacokinetic modeling approach. Not only does the parameter values correlate strongly, they were virtually identical. This suggests that we are not just measuring a model-specific parameter, but the actual physiological uptake rate.
We also showed that it is sufficient to acquire data up to only 10 minutes after Gadoxetate injection, in order to determine the hepatic uptake rate accurately. Finally, the use of a standard clinical imaging sequences is advantageous as the liver function measurement then easily can be incorporated into existing Gadoxetate-enhanced protocols.
Conclusion
Quantitative liver function measurements based on pharmacokinetic modeling of Gadoxetate‑MRI can be used fruitfully in a clinical setting. We have demonstrated the validity of the hepatic uptake rate estimation, which is easily incorporated into a standard Gadoxetate-enhanced protocol.Acknowledgements
No acknowledgement found.References
1. Sourbron S, et al. Combined quantification of liver perfusion and function with dynamic gadoxetic acid–enhanced MR imaging. Radiology 2012, 263(3):874-883.
2. Georgiou L, et al. Quantitative Assessment of Liver Function Using Gadoxetate-Enhanced Magnetic Resonance Imaging: Monitoring Transporter-Mediated Processes in Healthy Volunteers. Invest Radiol 2016, 52(2):111-119.
3. Forsgren MF, et al. Physiologically realistic and validated mathematical liver model revels hepatobiliary transfer rates for Gd-EOB-DTPA using human DCE-MRI data. PLoS ONE 2014, 9(4).
4. Ulloa JL, et al. Assessment of gadoxetate DCE‐MRI as a biomarker of hepatobiliary transporter inhibition. NMR in Biomedicine 2013, 26(10):1258-1270.
5. Leinhard OD, et al. Quantifying differences in hepatic uptake of the liver specific contrast agents Gd-EOB-DTPA and Gd-BOPTA: a pilot study. European radiology 2012, 22(3):642-653.
Figures