2619
Diagnostic value of intravoxel incoherent motion (IVIM) diffusion-weighted imaging in hepatic sinusoidal obstruction syndrome: an experimental study in a rat model – preliminary resultsEun Kyoung Hong1, Ijin Joo1, and Kyoungbun Lee2
1Department of Radiology, Seoul National University Hospital, Seoul, Republic of Korea, 2Department of Pathology, Seoul National University Hospital, Seoul, Republic of Korea
Synopsis
Hepatic sinusoidal obstruction syndrome (SOS), a toxic liver injury, needs an accurate diagnosis and serial monitoring for an effective management. Intravoxel incoherent motion (IVIM) DWI, which allows separate estimation of molecular diffusion and microcirculation, potentially provides information regarding hepatic parenchymal abnormalities. This study investigated the diagnostic value of IVIM-DWI in the assessment of hepatic SOS using a monocrotaline-induced rat SOS model. Our study results showed that ADC, true diffusion coefficient, and perfusion fraction showed significant correlation with the severity of SOS, which would suggest that IVIM-DWI may serve as a noninvasive method in the quantitative assessment of hepatic SOS.
Introduction
Hepatic sinusoidal obstruction syndrome (SOS), a toxic liver injury, is commonly caused by hematopoietic stem cell transplantation and systemic chemotherapy such as Oxaliplatin. An accurate diagnosis and serial monitoring of SOS would be needed for an effective management, and therefore, a noninvasive quantification of SOS is of great importance in clinical practice. Considering that hepatic SOS is characterized by damage to hepatocyte as well as hepatic sinusoids, it may affect the diffusion as well as perfusion features of the liver. Intravoxel incoherent motion (IVIM) diffusion-weighted imaging (DWI) allows separate estimation of molecular diffusion and microcirculation1; therefore, we hypothesize that IVIM-DWI can be useful in the diagnosis of hepatic SOS. Thus, the purpose of our study was to investigate the diagnostic value of IVIM-DWI in the severity assessment of hepatic SOS using a monocrotaline (MCT)-induced rat SOS model.Materials and methods
The institutional animal care and use committee approved this study. Twenty-four Sprague-Dawley rats (250-300g) were treated with vehicle (control group, n=6), 90 mg/kg of MCT (low-dose group, n=10), and 160 mg/kg of MCT (high-dose group, n=8). All rats underwent MRI 72 hours after treatment and were sacrificed immediately after MRI for histologic analysis. All MRI exams were performed with a 3.0-T MR imaging system, and IVIM-DWI were acquired by using a free-breathing single shot EPI with 9 b-values (0, 25, 50, 75, 100, 150, 200, 400, and 800 sec/mm2). ADC values were calculated by using all b values with a mono-exponential fit, and IVIM parameters including true diffusion coefficient (Dslow), pseudo-diffusion coefficient (Dfast), and perfusion fraction (PF) were calculated by using a nonlinear bi-exponential fit (Fig. 1). Five circular regions of interests (ROIs) were drawn for each ADC and IVIM parametric map at where it covers a large homogeneous area of liver, and the mean of five measurements for each parameter was used as a representative value. Based on the histologic features including sinusoidal hemorrhage, coagulation necrosis, and endothelial damage of the central vein2,3, each animal was categorized into one of the SOS severity groups: none, mild, moderate, and severe. The ADC and IVIM-DWI parameters were compared according to treatment groups and SOS severity groups using a Kruskal-Wallis test. In addition, those parameters were correlated with SOS severity using the using Spearman’s rank correlation test.Results
Among ADC and IVIM-DWI parameters, PF showed a significant difference according to treatment groups with medians of 17.2%, 12.9% and 10.8 % in control, low-dose MCT group, and high-dose MCT group, respectively (P<0.01). PF of hepatic parenchyma were significantly different according to SOS severity with medians of 17.9% in none (n=5), 11.9% in mild/moderate (n=6) and 9.9% in severe SOS (n=3) (P=0.01) (Fig. 2), and showed a strong negative correlation with SOS severity (rho=-0.84, P<0.01) (Table 1). In addition, ADC and Dslow showed a negative correlation with SOS severity with statistical significance (rho=-0.70 and -0.61, P<0.01 and 0.02, respectively) (Table 1).Discussion
Our study found that ADC, Dslow, and PF were significantly different according to the treatment groups and severity of hepatic SOS, and showed a negative correlation with SOS severity. These changes in diffusion- and perfusion-related parameters of IVIM-DWI may be explained by the histologic features of hepatic SOS including sinusoidal dilatation and congestion, fibrosis of the sinusoids and venules, and coagulation necrosis of hepatocytes that would potentially cause alterations in diffusion and perfusion characteristics of the liver4,5,6.Conclusion
IVIM-DWI may serve as a noninvasive method in the quantitative assessment of hepatic SOS.Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2015R1C1A2A01055423).References
1. Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 1988;168:497-505. 2. Wang X, Kanel GC, DeLeve LD. Support of sinusoidal endothelial cell glutathione prevents hepatic veno-occlusive disease in the rat. Hepatology 2000;31:428-34. 3. DeLeve LD, McCuskey RS, Wang X, et al. Characterization of a reproducible rat model of hepatic veno-occlusive disease. Hepatology 1999;29:1779-91. 4. Seo AN, Kim H. Sinusoidal obstruction syndrome after oxaliplatin-based chemotherapy. Clin Mol Hepatol 2014;20:81-4. 5. Kumar S, DeLeve LD, Kamath PS, Tefferi A. Hepatic veno-occlusive disease (sinusoidal obstruction syndrome) after hematopoietic stem cell transplantation. Mayo Clinic proceedings 2003;78:589-98. 6. Fontanilla T, Hernando CG, Claros JC, et al. Acoustic radiation force impulse elastography and contrast-enhanced sonography of sinusoidal obstructive syndrome (Veno-occlusive Disease): preliminary results. J Ultrasound Med 2011;30:1593-8.Figures
Table 1. Comparison of ADC and IVIM parameters according to histologic
severity of sinusoidal obstruction syndrome
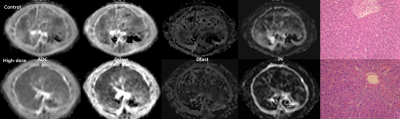
Figure 1. ADC,
Dslow, Dfast and PF maps and photographs of liver specimen (Hematoxylin-eosin
(H&E) stain, 200X) in rats (upper : control group, lower: monocrotaline
(MCT)-treated group, 160 mg/kg). On the H&E stained slides, different from
the normal liver of a control rat (upper), the liver specimen of a MCT-treated
rat (lower) demonstrates sinusoidal dilatation and hemorrhage, coagulation necrosis
of hepatocytes, and endothelial damage of the central vein, which are
characteristic findings of sinusoidal obstruction syndrome.
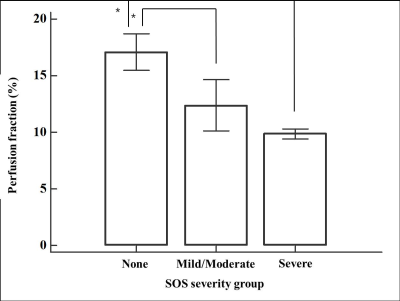
Figure 2. Bar
graphs show perfusion fraction for each SOS severity group. Bars represent the
median value and error bars represent a standard deviation. Asterisks represent
significant differences between SOS severity groups on post-hoc analysis (P
<0.05).