2616
Assessment of Liver fibrosis using Exchange dual-input dual-compartment pharmacokinetic model of Dynamic Contrast-enhanced MRI1MRI, The 1st affiliated hospital of Henan University of TCM, Zhengzhou, China, 2PHILIPS Healthcare, Beijing, China
Synopsis
The clinical need in the development of non-invasive methods for liver fibrosis assessment has emerged. At 3.0T, human in-vivo studies have demonstrated DCE-MRI using Exchange dual-input and dual-compartment pharmacokinetic model has potential to detect and assess the vascular permeability modification of liver fibrosis. DCE-MRI pharmacokinetic quantitative parameters including Ktrans, Ve and Vp can be used for diagnosing and staging liver fibrosis. Ktrans is the best index and predictor for discriminating normal livers from fibrotic livers.
Introduction
Liver fibrosis is an important cause of mortality and morbidity in patients with chronic liver disease. The end stage of liver fibrosis can eventually result in liver dysfunction. Early detection of liver fibrosis is essential, because it is reversible in early stage. While liver biopsy is the golden standard for liver fibrosis, inherent risk for invasive modality makes liver biopsy unusable for the clinical assessment and follow up. Some studies have focused on dynamic contrast-enhanced MRI (DCE-MRI) for assessment of liver fibrosis using a single-compartment model with some semi-quantitative parameters. Compared with single-compartment model, dual-input and dual-compartment model may be more suitable for assessment of microcirculation state in liver fibrosis. Exchange pharmacokinetic model with quantitative parameters can demonstrate the permeability modification, such as change of extravascular extracellular space (EES) and plasma space in a fibrotic liver. The purpose of this study was to evaluate the role of DCE-MRI with Exchange model in the assessment of liver fibrosis.Methods
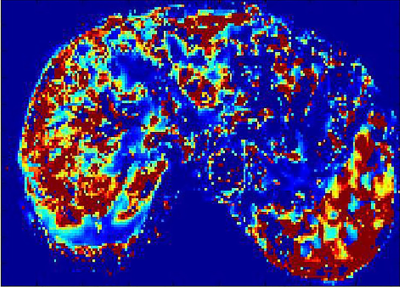
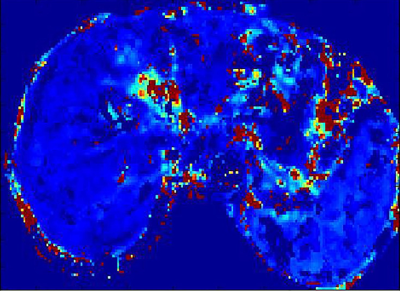
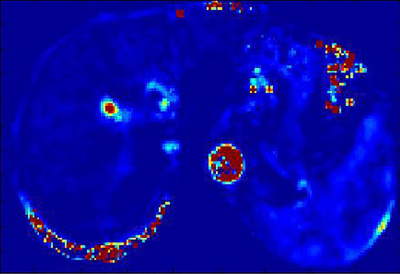
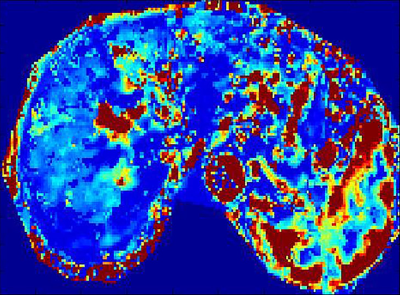
85 patients with chronic liver diseases confirmed by liver pathologic biopsy were prospectively enrolled. Live DCE-MRI with gadodiamide (Gd-DTPA-BMA) and 3D-THRIVE sequence was performed on a 3.0 T MRI scanner (Ingenia, Philips, Netherlands). MRI parameters were as follows: TR/TE,3.8/1.8ms;slice thickness, 3 mm; FOV 400mm×400mm; acquisition matrix 160mm×160mm; NSA 1; 30 images acquired for one phase, and 6.3s for each phase. The total acquisition time of 50 phases took about 5 min. Quantitative parameters were obtained by Exchange model (Omni-Kinetics, GE healthcare). The permeability parameters included Ktrans (volume transfer constant of the contrast agent), Kep (Reverse reflux rate constant), Ve (Volume fraction of EES) and Vp (Volume fraction of plasma). Standard hematoxylin-erosin staining and Maxon’s trichrome staing were used for staging liver fibrosis. Liver fibrosis was categorized for 5 stages, F0-F4. These categories were divided as the control group(F0), overall fibrosis group( F1-4), nonadvanced fibrosis group(F1-2), and advanced fibrosis group(F3-4). The t-test was used to evaluate the difference between the control and fibrotic group. One way analysis of variance was used to evaluate the differences among the control, nonadvanced and the advanced fibrosis group. The correlation between liver fibrosis stages and parameters was analyzed by Spearman rank test. A P value of less than 0.05 was considered significant.Results
The distribution of stages among 85 patients as follows: F0 ,n=20; F1,n=15; F2,n=15; F3,n=15; F4,n=20.Both Ktrans and Ve decreased as fibrosis stage increased. Ktrans of the control group was significantly different from that of the overall fibrosis group, the nonadvanced fibrosis group, and the advanced fibrosis group(p<0.01). As regards Ve, the control group was significantly different from the overall fibrosis group and advanced fibrosis group(p<0.01). Significant difference in Vp was found only between the control and advanced fibrosis group. There were no differences in Kep between the control , nonadvanced fibrosis, the advanced fibrosis groups. Fibrosis stage was negatively correlated with Ktrans and Ve (r=-0.69, p<0.01;r=-0.57, p<0.01). There were statistical differences between the area under the receiving operator characteristic curve (AUCOC) of Ktrans and that of Ve or Vp for differentiating between the control and overall fibrosis groups, between the control and nonadvanced fibrosis group, and between the control and advanced fibrosis groups. Overall, Ktrans was shown to be an excellent predictor among the quantitative parameters for differentiating normal livers from those with nonadvanced or advanced fibrosis.Discussion
The study has validated the feasibility of using Exchange model with quantitative parameters of DCE-MRI, including Ktrans, Ve, Vp, to assess liver fibrosis. Liver fibrosis hampers free exchange of low molecular compounds between the vascular space and interstitial space. Therefore, Ktrans demonstrated a decrease as liver fibrosis stage increased. Ktrans should decrease in a fibrotic liver due to the loss of normal fenestration. Morever, Ktrans had higher diagnostic performance for discriminating between normal and fibrotic livers than Ve. Ve also showed a decrease with increasing fibrosis stage, and was significantly lower in the overall fibrosis and advanced fibrosis groups than in the control group. Fibrosis-related cellular and molecular events may be responsible for this. Proliferation of fibrosis-related cells, such as hepatic stellate cells and myofibroblasts may result in decreased EES.Conclusions
DCE-MRI using Exchange dual-input and dual-compartment model could reflect variation of vascular microenvironment for liver fibrosis, and could be helpful to evaluate severity of and staging, suggesting quantitative parameters could be used as important indexes for the degree of liver fibrosis. Ktrans is an excellent predictor for differentiating fibrotic livers from normal livers, and differentiating normal livers from livers with nonadvanced or advanced fibrosis.Acknowledgements
Henan Foundation for Science and Technology Development (162102310104)References
[1] Mormone E, George J, Nieto N. Molecular pathogenesis of hepatic fibrosis and current therapeutic approaches[J]. Chem Biol Interact, 2011,193(3):225–231
[2] Eric Bultman, Ethan Brodsky, Debra K, et al. Quantitative hepatic perfusion modeling using DCE-MRI with sequential breath-Holds [J]. Magn Reson Imaging,2014,39(4): 853–865
[3] Shimon Aronhime, Claudia Calcagno, Guido Jajamovich, et al. DCE-MRI of the liver: effect of linear and non-linear conversions on hepatic perfusion quantification and reproducibility [J]. Magn Reson Imaging, 2014, 40(1): 90-98
[4] Sourbron S, Sommer WH, Reiser MF, et al. Combined quantification of liver perfusion and function with dynamic gadoxetic acid-enhanced MR imaging, Radiology,2012,263(3):874-883
[5] Li Z, Sun J, Chen L, et al. Assessment of liver fibrosis using pharmacokinetic parameters of dynamic contrast-enhanced magnetic resonance imaging[J]. Magn Reson Imaging, 2016, 44(1):98-104
[6] Leporq B, Dumortier J, Pilleul F, et al. 3D-liver perfusion MRI with the MS-325 blood agent: a noninvasive protocol to assess liver fibrosis. J Magn Reson Imaging, 2012,35(6):1380-1387
[7] Hagiwara M, Rusinek H, Lee VS, et al. Advanced liver fibrosis: diagnosis with 3D whole-liver perfusion MR imaging-initial experience [J]. Radiology, 2008,246(3):926-934
[8] Wang H, Cao Y. Correction of Arterial Input Function in Dynamic Contrast Enhanced MRI of the liver [J]. J Magn Reson Imaging,2012,36(2):411-42
Figures