2594
Assessment of Treatment Outcome in Chronic Hepatitis C Virus Infected Patients with Liver Stiffness Measured by Magnetic Resonance Elastography1Radiology, Charité - Universitätsmedizin Berlin, Berlin, Germany, 2Nephrology, Charité - Universitätsmedizin Berlin, Berlin, Germany, 3Pathology, Charité - Universitätsmedizin Berlin, Berlin, Germany, 4Medical Informatics, Charité - Universitätsmedizin Berlin, Berlin, Germany
Synopsis
High-resolution stiffness maps of the liver and kidney transplant (KTx) were generated after direct-acting antiviral therapy using multifrequency magnetic resonance elastography (MRE) and tomoelastography data processing in KTx recipients with chronic hepatitis C infection. Changes in liver stiffness after viral clearance were related to the immediate reduction in the inflammatory response in the early period and were stable until one year after end of treatment. MRE promises to be an early predictor for therapeutic success in HCV treatment.
Purpose:
To non-invasively monitor elastic properties of the liver and kidney transplants (KTx) using multifrequency magnetic resonance elastography (MRE) after direct-acting antiviral therapy.Background:
Hepatitis C virus (HCV) infection has a significantly higher prevalence in KTx recipients and is associated with increased morbidity and mortality. Novel direct-acting antiviral agents (DAA) are highly effective in complete viral clearance.Methods:
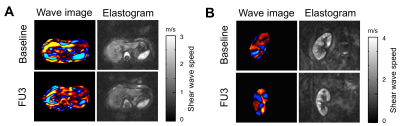
In this prospective and longitudinal study, 15 randomly selected KTx recipients with biopsy proven chronic HCV infection and with an estimated glomerular rate (eGFR) above 30 mL/min/1.73m2 were treated daily with Daclatasvir and Sofosbuvir for 3 months. Shear wave speed (SWS) of the liver and KTx was measured by multifrequency MRE (1) at baseline (BL), the end of therapy (EOT), and 3 (FU3) and 12 months follow-up (FU12) after EOT. The results were compared to a healthy control group (CTR, n=7). All experiments were conducted on a 1.5-T MRI scanner equipped with a 12-channel phased array surface coil. Mechanical vibrations were induced by three pressurized air driven actuators for liver and single actuator for KTx. The frequency range of mechanical vibration were 30 to 60 Hz for liver and 40 to 70 Hz for KTx. Imaging parameters were the same as described in (2). MRE imaging protocols were executed in an axial (liver) and paracoronal (KTx) slice orientation with free breathing. MRE data post-processing was based on the tomoelastography pipeline detailed in (3), yielding wave speed maps (elastograms) in m/s. Laboratory results were obtained at each time point, corresponding to MRE.Results:
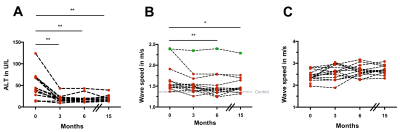
Mean time to reach undetectable viral RNA was 22±13 days. 14 of 15 patients had undetectable viral RNA at EOT and FU3. Alanine aminotransferase (ALT) values decreased significantly (P<0.01) in all patients after treatment at EOT (19.3±8.3 U/L) compared with BL (50.6±29.8 U/L) and remained unchanged at FU3 (20.2±9.3 U/L) and FU12 (19.5±7.3 U/L) (see Fig. 2A). Analysis of repeated measurements showed a significant decrease in liver SWS (stiffness) at FU3 compared with BL (BL: 1.67±0.33 m/s vs. FU3: 1.52±0.29 m/s; P=0.002) and remained stable in long-term follow-up at FU12 (1.55±0.26 m/s; P<0.05) in all patients. Viral relapse occurred in one patient and was only detected with MRE showing unchanged liver SWS (see Fig. 1A and 2B). Longitudinal increase in group-SWS was observed in KTx (see Fig. 1B and 2C).Discussion:
In this prospective study, tomoelastography was used for the first time to assess the short- and long-term outcome of DAA treatment in HCV infected patients. In combination with our observation that ALT has returned to normal level at EOT (fig. 2A), we hypothesize that the softening tendency of the liver at this early time could be related to the reduction of interstitial edema which often arises in response to inflammatory processes. A significant reduction in liver SWS compared with baseline was observed at FU3. Additionally, the liver SWSs of the patients at this time were comparable to the ones of healthy controls, while being significantly different at BL and EOT. Follow-up after one year (FU12) provided stable SWS-values compared with FU3, which was significantly lower than at BL (fig. 2B). As the patients' ALT became normal at EOT and remained stable afterwards (fig. 2A), further reduction in liver SWS at FU3 could be related to another process such as "remodeling" as reported in (4). Interestingly, in one patient who developed a viral relapse, a unique non-responsiveness of liver SWS was observed after treatment (fig. 2B). Such behavior was observed neither in HCV-RNA load, nor in ALT, nor in both serological fibrosis scores. Although a slight increase of renal SWS was observed longitudinally, post-hoc analyses showed no change in SWS between any time points (fig. 2C). This variation in renal SWS could be related to changes in perfusion as a result of DAA, as previously published reports on the dependency of renal SWS to perfusion pressure in an ex vivo model (5).Conclusion:
Changes in liver stiffness after viral clearance were related to the immediate reduction in the inflammatory response in the early period and were stable until one year after end of treatment. MRE promises to be an early predictor for therapeutic success in HCV treatment.Acknowledgements
The author would like to thank the Federal Ministry for Education and Research for their financial support (BMBF LiSym-031L0057 to IS).References
1. Muthupillai R, Ehman RL. Magnetic resonance elastography. Nat Med 1996;2(5):601-603.
2. Dittmann F, Tzschatzsch H, Hirsch S, Barnhill E, Braun J, Sack I, Guo J. Tomoelastography of the abdomen: Tissue mechanical properties of the liver, spleen, kidney, and pancreas from single MR elastography scans at different hydration states. Magn Reson Med 2017;78(3):976-983.
3. Tzschatzsch H, Guo J, Dittmann F, Hirsch S, Barnhill E, Johrens K, Braun J, Sack I. Tomoelastography by multifrequency wave number recovery from time-harmonic propagating shear waves. Med Image Anal 2016;30:1-10.
4. Shiratori Y, Imazeki F, Moriyama M, Yano M, Arakawa Y, Yokosuka O, Kuroki T, Nishiguchi S, Sata M, Yamada G, Fujiyama S, Yoshida H, Omata M. Histologic improvement of fibrosis in patients with hepatitis C who have sustained response to interferon therapy. Ann Intern Med 2000;132(7):517-524.
5. Marticorena Garcia SR, Grossmann M, Lang ST, Tzschatzsch H, Dittmann F, Hamm B, Braun J, Guo J, Sack I. Tomoelastography of the native kidney: Regional variation and physiological effects on in vivo renal stiffness. Magn Reson Med 2017.
Figures