2588
11-Second Hepatic MR Elastography in Clinical Research Trials1200 1st St Sw, Mayo Clinic, Rochester, MN, United States, 2Florida International University, Miami, FL, United States, 3Siemens Healthineers, Salt Lake City, UT, United States
Synopsis
As a non-invasive imaging technique for detecting and staging liver fibrosis, MR Elastography (MRE) is highly sensitive and specific. Conventional 2D liver GREMRE is very effective, and only takes about 1-2 minutes with multiple breath-holds (11-16 seconds, each). However, shorter acquisition times and fewer breath-holds are always desired for these examinations, especially when patients have difficulty holding their breath. In this study, we developed an 11-second hepatic MRE protocol based on SE-EPIMRE sequence, which was performed in a single breath-hold comfortably; the repeatability of repeated MRE scans was also assessed.
INTRODUCTION
As a non-invasive imaging technique for detecting and staging liver fibrosis, MR Elastography (MRE) is highly sensitive and specific (both rates: 85%-100%), using liver biopsy as a reference method 1. Conventional 2D liver GREMRE is very effective, and only takes about 1-2 minutes with multiple breath-holds (11-16 seconds, each) 2. However, shorter acquisition times and fewer breath-holds are always desired for these examinations, especially when patients have difficulty holding their breath. We have recently developed an 11-second hepatic MRE protocol based on SE-EPIMRE sequence, which requires only a single 11 second breath-hold.
Liver fibrosis is a common consequence of all liver injuries caused by hepatitis viruses (B, C), alcohol use, and/or other etiologies, and is one of the major causes of death in HIV infected people 3-5. We tested the 11-second hepatic MRE protocol in a clinical study to determine liver fibrosis progression in HIV infected patients who use or do not use cocaine. The purpose was to evaluate the efficacy of this 11-second MRE protocol. Our hypothesis is that this protocol can be comfortably completed within one breath-hold, and that liver stiffness measurements are highly reproducible when MRE is repeated.
METHODS
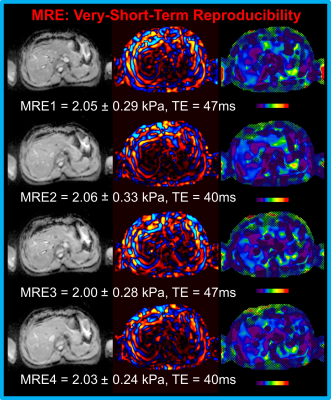
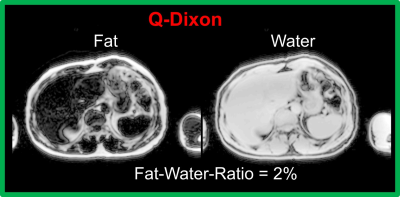
11-second MRE protocol: The prototype SE-EPIMRE acquisition (11 seconds) was scanned 4 times (very-short-term test-retest) with the following major parameters: TR=1000 ms, TE = 47 or 40 ms corresponding to fractional motion encoding gradient = 100% or 80%, FOV = 42 cm, slice thickness/spacing = 6 mm/7.2mm, acquisition matrix = 100×100. Between the first two (TE = 47, 40ms) and last two MREs (TE = 47, 40ms). In addition, a VIBE Q-DIXON (5 echoes) sequence (16 seconds) was scanned for liver fat-water-ratio evaluation with the following major imaging parameters: TR = 9ms, FOV = 42/37cm, slice thickness = 3 mm, acquisition matrix = 160×140. Patients were in head-first supine position; all the scans were performed at the end of expiration on a MAGNETOM Prisma 3T scanner (Siemens Healthineers, Erlangen, Germany).
Patients: The 11-second MRE protocol was tested in the Miami Adult Studies on HIV (MASH) patients 6,7, who are HIV-infected, HIV/HCV co-infected, HCV-mono-infected, or HIV/HCV/HBV un-infected. More than 40% MASH patients use cocaine. This study has been approved by IRB at FIU. This abstract only reports the first 11 patients; additional patients are undergoing MREs and will be analyzed as the study moves forward.
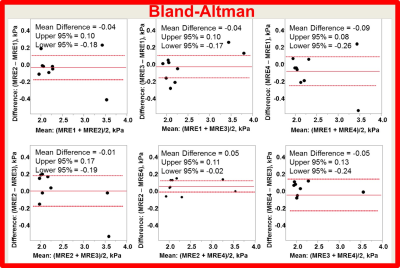
Reproducibility of MRE: intra-class correlation and Bland-Altman were analyzed between stiffness measurements with all 4 MREs by using JMP 12 Pro (Cary, NC).
RESULTS
All 11 patients completed the 11-sec MRE protocol within one breath-hold, comfortably. Fig. 1 shows examples of the 11-sec MRE images. Mean liver stiffness ranged from 1.97 kPa to 3.55 kPa. The Bland-Altman shows that the mean difference between any two MREs ranged from 0.01 to 0.09 kPa (Fig. 2). The intra-class correlation was 0.95 (1.0 means measurements were identical) among the four MREs. Fig. 3 shows examples of the fat and water images collected by using VIBE Q-Dixon. The fat-water-ratio ranged from 1% to 9%.
CONCLUSION
The patients completed the 11-sec hepatic MRE protocol within one breath-hold comfortably. The liver stiffness measurements using this protocol are highly reproducible in the 4 repeated 11-second MRE acquisitions.Acknowledgements
No acknowledgement found.References
1. Huwart L, Sempoux C, Vicaut E, et al. Magnetic resonance elastography for the noninvasive staging of liver fibrosis. Gastroenterology. 2008;135(1):32-40.
2. Venkatesh SK, Ehman RL. Magnetic resonance elastography of liver. Magnetic resonance imaging clinics of North America. 2014;22(3):433-446.
3. Smith C, Sabin CA, Lundgren JD, et al. Factors associated with specific causes of death amongst HIV-positive individuals in the D:A:D Study. AIDS. 2010;24(10):1537-1548.
4. van der Helm J, Geskus R, Sabin C, et al. Effect of HCV infection on cause-specific mortality after HIV seroconversion, before and after 1997. Gastroenterology. 2013;144(4):751-760 e752.
5. Adih WK, Selik RM, Hu X. Trends in Diseases Reported on US Death Certificates That Mentioned HIV Infection, 1996-2006. Journal of the International Association of Physicians in AIDS Care. 2011;10(1):5-11.
6. Baum MK, Jayaweera DT, Duan R, et al. Quality of life, symptomatology and healthcare utilization in HIV/HCV co-infected drug users in Miami. Journal of addictive diseases. 2008;27(2):37-48.
7. Campa A, Martinez SS, Sherman KE, et al. Cocaine Use and Liver Disease are Associated with All-Cause Mortality in the Miami Adult Studies in HIV (MASH) Cohort. J Drug Abuse. 2016;2(4).