2575
Voxel Level Radiologic–Pathologic Validation of DCE-MRI with ISUP Grade in Prostate Cancer1Department of Urology, Drum Tower Hospital, Medical School of Nanjing University, Institute of Urology, Nanjing University, Nanjing, China, 2Department of Radiology, Drum Tower Hospital, Medical School of Nanjing University, Nanjing, China, 3Department of Pathology, Drum Tower Hospital, Medical School of Nanjing University, Nanjing, China, 4United Imaging Healthcare Co., Ltd, Shanghai, China, 5GE Healthcare, Nanjing, China
Synopsis
The biggest challenge in patients with newly diagnosed PCa is shifting from cancer detection or staging alone to identifying them with aggressive disease. The PI-RADS v 2 recognizes the role of DCE-MRI is limited but is essential. This work presented a radiology pathology correlation framework that enabled identification of promising in vivo DCE MRI markers of PCa risk at voxel level. The relationship between ISUP grade and DCE-MRI (Ktrans and Kep) suggests that it may be used as a component of active surveillance to noninvasively detect high-grade PCa and affect staging and treatment.
Introduction
The goals of prostate cancer (PCa) diagnosis are detecting, staging, and characterizing the biologic aggressiveness as a prognostic marker[1]. The biggest challenge in patients with newly diagnosed PCa is shifting from cancer detection or staging alone to identifying those patients with aggressive disease who would benefit from more radical therapy, while sparing those with indolent cancers[2]. The Prostate Imaging Reporting and Data System (PI-RADS) version 2 recognizes that the evidence supporting the role of dynamic contrast enhanced (DCE) magnetic resonance imaging (MRI) is limited, and that the added value provided by DCE MRI appears to be modest[3]. Nonetheless, PI-RADS version 2 recommends that DCE be included in MRI scans, describing the DCE sequence as essential. Prior reports show that the quantitative signal derived from DCE MRI is associated with aggressive PCa as measured by ISUP grade[4]. We evaluated the reliability of DCE MRI to predict variance with ISUP grade at the voxel-level within clinically demarcated PCa regions.Experimental Design
Thirteen patients (age: 67.31±9.16 year) were
scanned, given institutional ethics approval and informed written consent, on a
3.0T MR scanner system (MR770, UIH, Shanghai, China). The DCE protocols were
used to obtain the precontrast T1map and dynamic contrast data with 3D SPGR sequence,
TR=4.3ms, TE=1.8ms, 28 slices, slice thickness 2mm, FOV 28x38cm2, resolution 306x416,
BW=450Hz. T1map scan was based on VFA method with FA=15/12/9/6/3 degrees. The
dynamic scan was performed for 75 phases with temporal resolution of 4.7 sec, scan
time=5’50’’ in total, administration of 0.1 mmol/kg GD-DPTA at the beginning of
dynamic scan, using FA=10 degree. The Pharmokinetic parameters including Ktrans
and Kep for prostate were obtained by processing the presurgical DCE MRI data
using the extended Tofts model. All DCE MRI data were processed using whole
mount sectioning after radical prostatectomy. Regions of tumor were identified by an uropathologist.
Stained prostate sections were scanned at high resolution (75 mm/pixel). A grid
of tiles corresponding to voxel dimensions was graded using the ISUP grade
system (Fig. 1).Results
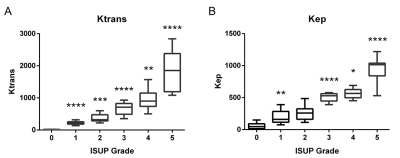
Ktrans and Kep
dirived from DCE-MRI were found to distinguish between PCa
and benign tissue. Significant differences were also found between benign
tissue (ISUP 0 Grade) and PCa classified as low-grade or high-grade (Fig. 2).Conclusions
This work presented a radiology pathology correlation framework that enabled identification of promising in vivo DCE MRI markers of PCa risk. The relationship between ISUP grade and DCE-MRI (Ktrans and Kep) suggests that DCE-MRI may be used as a component of active surveillance to noninvasively detect high-grade PCa and affect staging and treatment. Furthermore, because it can detect variations in tumor grade with voxel-level precision, DCE-MRI may be a promising option for planning of focal procedures, such as MRI-guided targeted biopsies and targeted radiotherapy, where identifying the area with the most aggressive disease is particularly important. Moreover, the MRI images matched to histopathology sections were selected by experience, and automatic algorithm image matching will be better in our future work.Acknowledgements
No acknowledgement found.References
[1] van Niekerk CG, van der Laak JA, Hambrock T, et al. Correlation between dynamic contrast-enhanced MRI and quantitative histopathologic microvascular parameters in organ-confined prostate cancer. Eur Radiol. 2014. 24(10): 2597-605.
[2] Guneyli S, Erdem CZ, Erdem LO. Magnetic resonance imaging of prostate cancer. Clin Imaging. 2016. 40(4): 601-9.
[3] Weinreb JC, Barentsz JO, Choyke PL, et al. PI-RADS Prostate Imaging - Reporting and Data System: 2015, Version 2. Eur Urol. 2016. 69(1): 16-40.
[4] Epstein JI, Zelefsky MJ, Sjoberg DD, et al. A Contemporary Prostate Cancer Grading System: A Validated Alternative to the Gleason Score. Eur Urol. 2016. 69(3): 428-35.
Figures