2571
Ex vivo ultra-high-field 9.4-Tesla magnetic resonance elastography (MRE) in comparison to whole-mount pathology for improved prostate cancer diagnostics.1Richard and Loan Hill Department of Bioengineering, University of Illinois at Chicago, Chicago, IL, United States, 2Department of Radiology, Charité - Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Berlin, Germany, 3Department of Urology, University of Illinois at Chicago, Chicago, IL, United States, 4Department of Radiology, University of Illinois at Chicago, Chicago, IL, United States, 5Department of Pathology, University of Illinois at Chicago, Chicago, IL, United States
Synopsis
Despite the success of multiparametric magnetic resonance imaging (mpMRI) for the assessment of prostate cancer, it suffers from limitations such as a moderate inter-reader reliability and sub-optimal diagnostic accuracy. This is the first study for the assessment of 6 human prostate specimens without pathology fixation or prior radiation therapy using ex vivo 9.4-Tesla magnetic resonance elastography (MRE). Using whole-mount pathology as a reference, preliminary results show a sensitivity and specificity of 86 % and 52 %, respectively. MRE has the potential to improve the differentiation of benign prostatic hyperplasia nodules from malignant lesions, which is a known limitation of mpMRI.
Purpose
To assess the detection and localization of prostate cancer (PCa) using an ex vivo ultra-high-field 9.4-Tesla magnetic resonance elastography (MRE) setup. Despite the success of multiparametric magnetic resonance imaging (mpMRI) for the assessment of prostate cancer (PCa), it suffers from limitations such as a moderate inter-reader reliability, low sensitivity to detect extraprostatic tumor extension and low specificity to differentiate benign prostatic hyperplasia (BPH) nodules from malignant lesions [1,2]. An additional MRE sequence might improve the diagnostic accuracy and preoperative planning. In contrast to previous ex vivo MRE studies, the prostate specimens in this study were examined without pathology fixation or prior radiation therapy, which are procedures that influence the mechanical tissue properties [3–5].Methods
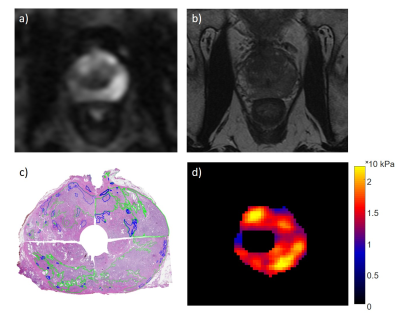
Immediately after radical prostatectomy, 6 human prostate specimens underwent MRE using a pre-clinical ultra-high-field 9.4-Tesla MRI (Agilent, 310/ASR, Santa Clara, California, USA). The period from the operation room to pathology was 2 h or less. A modified spin-echo pulse sequence based on sample interval modulation (SLIM)-MRE for the simultaneous acquisition of the wave field displacement along all three Cartesian directions was used at 500 Hz (Fig. 1) [6,7]. MRE data were acquired with a matrix of 64 x 64, a field of view of 64 x 64 mm and 1 mm slice thickness. A region of interest was generated from the transversal magnitude images by manual segmentation of the prostate contour and urethra. The prostate was divided into 12 segments (right/left, base/mid/apex, anterior/posterior) for both MRE and whole-mount pathology. The results were correlated to determine the sensitivity and specificity of MRE parameters.Results
According to pathology, 33 of the total 72 segments and an average of 5.5 of the 12 segments for each prostate were positive for PCa. For MRE, a threshold based PCa identification method was used. A segment was defined as cancerous when the stiffness values of 10 % of its pixels were higher than the mean stiffness (G') + standard deviation (SD) (averaged over the whole ex vivo prostate). G' ± SD for all segments, healthy segments, and cancerous segments were found to be 6.15 ± 1.04 kPa, 4.20 ± 0.59 kPa, and 6.44 ± 1.06 kPa, respectively. Sensitivity and specificity were 86 % and 52 %, respectively.Discussion
This study shows promising preliminary results for the assessment of PCa of ex vivo human specimens without fixation or prior radiation therapy. In comparison to a current mpMRI study, ex vivo MRE was slightly less sensitive (93 % vs. 86 %) but more specific (41 % vs. 52 %) for cancer detection [2]. For the calculation of diagnostic accuracy, specimen No. 6 had to be excluded because all segments were cancerous according to pathology and, therefore, the threshold method could not be used. The examination of more prostate specimens is planned. In conclusion, MRE has the potential to add valuable information to current scan protocols and to improve the differentiation of BPH nodules from malignant lesions, which is a known limitation of mpMRI.
Acknowledgements
No acknowledgement found.References
[1] Weinreb JC, Barentsz JO, Choyke PL, et al. PI-RADS Prostate Imaging - Reporting and Data System: 2015, Version 2. Eur Urol. 2016;69(1):16–40.
[2] Ahmed HU, El-Shater Bosaily A, Brown LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet. 2017;389(10071):815–822.
[3] McGrath DM, Lee J, Foltz WD, et al. MR elastography to measure the effects of cancer and pathology fixation on prostate biomechanics, and comparison with T1, T2 and ADC. Phys Med Biol. 2017;62(3):1126–1148.
[4] Sahebjavaher RS, Nir G, Gagnon LO, et al. MR elastography and diffusion-weighted imaging of ex vivo prostate cancer: Quantitative comparison to histopathology. NMR Biomed. 2015;28(1):89–100.
[5] Dresner MA, Cheville JC, Myers RP, et al. MR Elastography of Prostate Cancer. Proc Intl Soc Mag Reson Med. 2003;11:2003.
[6] Kearney SP, Majumdar S, Royston TJ, et al. Simultaneous 3D MR elastography of the in vivo mouse brain. Phys Med Biol. 2017;62(19):7682-7693.
[7] Klatt D, Yasar TK, Royston TJ, et al. Sample interval modulation for the simultaneous acquisition of displacement vector data in magnetic resonance elastography: theory and application. Phys Med Biol. 2013;58(24):8663–8675.
Figures