Alireza Ziaei1, Francesco Alessandrino1,2, Mark Vangel3, Tina Kapur1, Clare Mary Tempany1, and Fiona Mary Fennessy1,2
1Dept. of Radiology, Harvard Medical School, Brigham and Women's Hospital, Boston, MA, United States, 2Dept. of Imaging, Dana–Farber Cancer Institute, Boston, MA, United States, 3Dept. of Radiology, Harvard Medical School, Massachusetts General Hospital, Charlestown, MA, United States
Synopsis
The aim of this
retrospective study was to determine a role for PIRADS V2 in conjunction with
TRUSgBX to predict the presence of clinically significant prostate cancer
(csPCa) in treatment naïve men with pathology-proven prostate cancer who
underwent TRUSgBX, followed by 3T mp-MRI prostate, and subsequently underwent
RP. Our
findings suggest that adding PIRADS V2 assessment to TRUSgBX improves the prediction of
final pathology for presence of indolent disease and csPCa, and
may help alleviate the rate of upgrading at RP.
INTRODUCTION
Transrectal ultrasound guided prostate biopsy
(TRUSgBX) is a common
diagnostic test for prostate cancer (PCa), which is known to have significant
limitations [1-4]. Multiparametric
MRI (mp-MRI) guided target biopsy methods have been shown to out-perform TRUSgBX
for detection and diagnosis of clinically significant PCa (csPCa) [5-7],
defined as presence of Gleason pattern > 4 and/or tumor volume>
0.5 ccs, and to reduce detection of indolent cancers [8]. We sought
to determine the role of PIRADS V2 in conjunction with TRUSgBX to predict
presence of csPCa.MATERIALS AND METHODS
This study was an IRB approved, HIPAA compliant,
retrospective study of treatment naïve men with pathology-proven PCa who
underwent TRUSgBX, followed by 3T mp-MRI prostate, and subsequently underwent radical
prostatectomy (RP) within 6 months of mp-MRI. A PIRADS V2 assessment category
was assigned by a radiologist blinded to all pathology. For this study we
defined a positive score of 4 or 5 as indicating csPCa. PIRADS V2 assessment
for csPCa and TRUSgBX pathology were compared to reference RP pathology. The
statistical tests were performed using the SPSS 15.0 (SPSS Inc., Chicago,
Illinois, USA) software, with p < 0.05 signifying statistical significance.RESULTS
Our
cohort consisted of 208 men, with a mean age of 59±7 years, and mean PSA level
of 6.7±4.6 ng/ml. The majority (94%) of patients underwent mp-MRI after TRUSgBX
(1.3 months median time interval), and went to RP within 1.7 months (median
time) of the mp-MRI. Final pathology documented GS ≥ 7 in 155/208 cases (74.5%). On
average, there were 12 samples/patient for TRUSgBX, with mean positive core
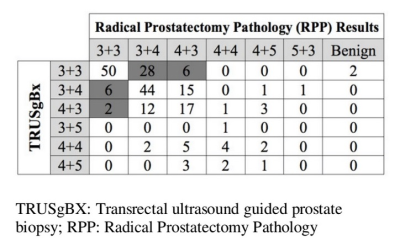
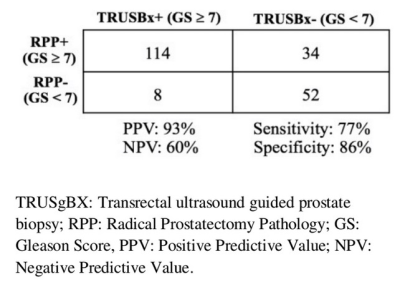
involvement of 4.3/12, range of positive core was1 to 14. Pre-operative TRUSgBX revealed 127/208 (61%)
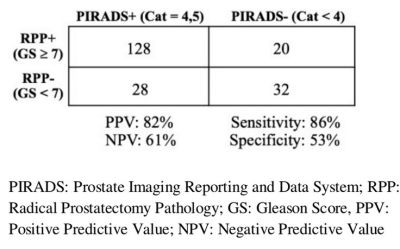
of subjects to have csPCA. Pre-operative PIRADS V2 assessment category 4/5 was
reported in 156/208 (75%) cases. TRUS failed to detect csPCa in 34/208 (16.34%)
cases, with subsequent upstaging to csPCa at final pathology (Table 1). There
were 8/208 cases considered csPCa at TRUSgBX, who ultimately had indolent
disease at final pathology. Of the 34 TRUSgBX underdiagnosed
cases, 28 (82%) were correctly diagnosed as csPCa with PIRADS V2, and 6 were not identified either. Of the 8 TRUSgBX-overdiagnosed
cases, 6 (75%) were correctly diagnosed as indolent with PIRADS V2. PIRADS V2 was more sensitive than TRUSgBX
(86.5% vs. 77% respectively, p = 0.04) for csPCa detection. TRUSgBX was more
specific than PIRADS V2 (86.7% vs. 53.3%, p < 0.001) (Table 2 and 3). TRUSgBX
had a higher Positive Predictive Value (PPV) compare to PIRADS V2 (93% vs. 82%
respectively). Negative Predictive Value (NPV)s for TRUSgBX and PIRADS V2 were
60% and 61% respectively.DISCUSSION
Our study demonstrates a potential role for PIRADS V2 assessment in
conjunction with TRUSgBX, for increased sensitivity in detection of both
indolent and csPCa, with a potential for reduction in TRUSgBX-underdiagnosis
and and overdiagnosis of csPCa. PIRADS V2
failed to detect a small number (6/34) of csPCa cases that were also not diagnosed by TRUSgBX. These findings suggest
that adding PIRADS V2 assessment to TRUSgBX increases the sensitivity for csPCa
detection prior to RP, and may alleviate the rate of upgrading at RP. However,
this should be evaluated in a prospective clinical trial. It is important to note that our study has some inherent
biases, as it focused on a surgical population of men with known PCa. As such,
this selection bias increased the probability of having csPCa, which affected
both the PPV and NPV.CONCLUSION
A combination
of TRUSgBX and PIRADS V2 assessment improves the prediction of final pathology
for presence of indolent disease and csPCa, compared to TRUSgBX alone.Acknowledgements
Research reported in this abstract is supported
by NIH Grants No. R25CA089017 and P41EB015898.References
1.
Hassanzadeh E, Glazer DI, Dunne RM, Fennessy FM, Harisinghani MG, Tempany CM.
Prostate imaging reporting and data system version 2 (PIRADS V2): a pictorial
review. Abdominal radiology (New York). 2017 Jan;42(1):278.
2.
Schoots IG, Roobol MJ, Nieboer D, Bangma CH, Steyerberg EW, Hunink MM. Magnetic
resonance imaging–targeted biopsy may enhance the diagnostic accuracy of
significant prostate cancer detection compared to standard transrectal
ultrasound-guided biopsy: a systematic review and meta-analysis. European
urology. 2015 Sep 30;68(3):438-50.3. Epstein JI, Feng Z, Trock BJ, Pierorazio
PM. Upgrading and downgrading of prostate cancer from biopsy to radical
prostatectomy: incidence and predictive factors using the modified Gleason
grading system and factoring in tertiary grades. European urology. 2012 May
31;61(5):1019-24.
4.
Penzkofer T, Tuncali K, Fedorov A, Song SE, Tokuda J, Fennessy FM, Vangel MG,
Kibel AS, Mulkern RV, Wells WM, Hata N. Transperineal in-bore 3-T MR
imaging–guided prostate biopsy: a prospective clinical observational study.
Radiology. 2014 Sep 15;274(1):170-80.
5.
Turkbey B, Pinto PA, Mani H, Bernardo M, Pang Y, McKinney YL, Khurana K,
Ravizzini GC, Albert PS, Merino MJ, Choyke PL. Prostate cancer: value of
multiparametric mr imaging at 3 t for detection—histopathologifc correlation 1.
Radiology. 2010 Mar 10;255(1):89-99.
6.
Soylu FN, Eggener S, Oto A. Local staging of prostate cancer with MRI.
Diagnostic and Interventional Radiology. 2012 Jul 1;18(4):365.
7.
Cornud F, Flam T, Chauveinc L, Hamida K, Chrétien Y, Vieillefond A, Hélénon O,
Moreau JF. Extraprostatic spread of clinically localized prostate cancer:
factors predictive of pT3 tumor and of positive endorectal MR imaging
examination results. Radiology. 2002 Jul;224(1):203-10.
8.
Wolters T, Roobol MJ, van Leeuwen PJ, et al. A critical analysis of the tumor
volume threshold for clinically insignificant prostate cancer using a data set
of a randomized screening trial. J. Urol. 2011;185(1):121–125.
9.
Borofsky S, George AK, Gaur S, Bernardo M, Greer MD, Mertan FV, Taffel M,
Moreno V, Merino MJ, Wood BJ, Pinto PA. What Are We Missing? False-Negative
Cancers at Multiparametric MR Imaging of the Prostate. Radiology. 2017 Oct
20:152877.
10. Vargas HA, Hötker AM, Goldman
DA, Moskowitz CS, Gondo T, Matsumoto K, Ehdaie B, Woo S, Fine SW, Reuter VE,
Sala E. Updated prostate imaging reporting and data system (PIRADS V2)
recommendations for the detection of clinically significant prostate cancer
using multiparametric MRI: critical evaluation using whole-mount pathology as
standard of reference. European radiology. 2016 Jun 1;26(6):1606-12.
11. Ahmed HU, Bosaily
AE, Brown LC, Gabe R, Kaplan R, Parmar MK, Collaco-Moraes Y, Ward K, Hindley
RG, Freeman A, Kirkham AP. Diagnostic accuracy of multi-parametric MRI and TRUS
biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. The
Lancet. 2017 Mar 3;389(10071):815-22.