2557
Whole-body MRI for prostate cancer at primary staging: interobserver concordance, diagnostic accuracy and protocol optimisation1Centre for Medical Imaging, University College London, London, United Kingdom
Synopsis
Whole body (WB) MRI is developing as a cancer staging platform in primary prostate cancer, although has not yet been adopted into clinical practice. In this study, we show that WB-MRI provides high levels of interobserver concordance, intermodality concordance and diagnostic accuracy for both nodal and metastatic bone disease, with higher levels of sensitivity than BS for metastatic disease, and similar performance to PET/CT. We also show that T2W and post contrast mDixon have no additive diagnostic value above T1W and DWI alone.
INTRODUCTION
The mainstay of imaging-based staging decisions are still dependent upon bone scan (BS) +/- pelvic CT, as supported by at least eight international guidelines 1. Whilst such modalities are simple to implement, their performance characteristics are limited 2,3 and have not been fully addressed by the inception of choline PET/CT.
Here, we seek to determine the interobserver concordance of multi parametric (mp)-whole body (WB)-MRI and compare lesion distribution and intermodality concordance with BS and PET/CT for the primary staging of intermediate and high-risk prostate cancer. The diagnostic accuracy of each modality will also be assessed using a one-year follow-up mp-WB-MRI based reference standard and a locked sequential read (LSR) paradigm to determine the additive value of each MRI sequence.
METHODS
56 consecutive men (mean age 67.9 years, range 51.9 – 84.4) were identified between July 2012 and November 2015. Standard imaging comprised BS in all patients +/- 18F-choline-PET/CT in 33 patients.
MRI protocol:
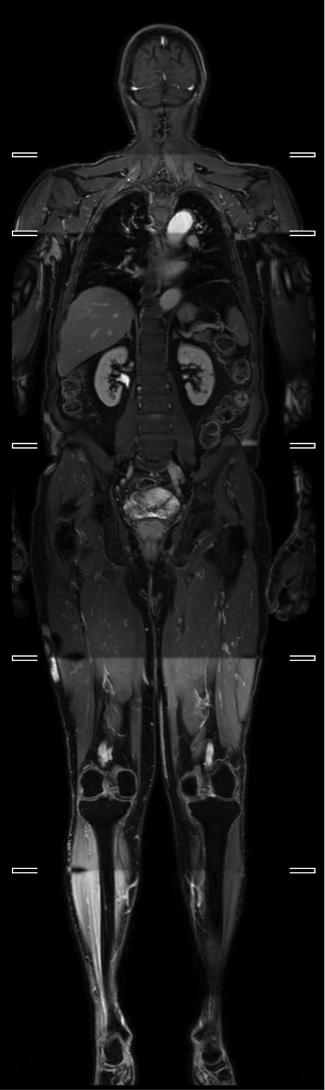
All patients were imaged on a 3.0 T wide bore system (Ingenia, Philips, Best, NL), with WB coverage from vertex to feet using a 6 station acquisition, a head coil, two anterior surface coils and table-embedded posterior coils. Coronal pre-contrast mDixon, axial T2W TSE and axial DWI at 4 b-values (b0, b100, 300 and 1000) were acquired, and an ADC map was constructed. Post-contrast mDixon imaging was carried out following a 20ml injection of intravenous gadolinium (Figure 1).
Follow-up WB-MRI:
Patients were invited to attend a follow-up WB-MRI 1 year after their initial scan – using the same acquisition protocol to inform the reference standard. 29 patients attended in total.
WB-MRI review:
Three board certified radiologists reviewed scans using a locked sequential read (LSR) paradigm, whereby each radiologist scored the suspicion of disease at each site using a 1 – 6 ordinal scale (1; definitely not present, 2; probably not present, 3; possibly not present, 4; possibly present, 5; probably present, 6; definitely present) for according to the ‘TNM’ 7th edition staging system (N0/N1, M1a/M1b/M1c). T1 and DWI were reviewed first, then T2 added, then post contrast Dixon.
Reference standard:
True positive (TP) sites: i) Lesion on WB-MRI (defined as suspicion level 4/5/6) which is BS and 18F-choline-PET/CT positive (if performed). Follow up WB-MRI (if performed) also demonstrates lesion progression without systemic therapy, decrease with systemic therapy, or new lesions. ii) Lesion on WB-MRI which is BS or PET/CT negative (if performed) but progresses on WB MRI follow up without systemic therapy, decreases with systemic therapy, or new lesions identified.
True negative (TN) sites: No lesion on WB MRI (defined as suspicion level 1/2/3) or BS or PET/CT, unchanged at follow up and without evidence of biochemical failure, as per the Phoenix definition 4.
False positive (FP) sites: Lesion on WB-MRI that was BS or PET/CT negative and unchanged at follow-up up. No evidence of biochemical failure.
False negative (FN) sites: No lesion on WB MRI but positive BS or PET/CT, which increased without, or decreased in size with systemic therapy at follow-up.
Statistical analysis
The following statistics were calculated:
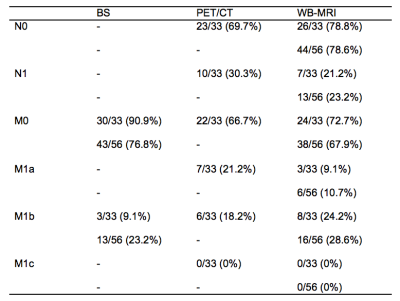
1. Positive lesion distribution for each staging modality (BS, PET/CT and WB-MRI) for local nodal and metastatic disease using the ‘TNM’ classification. Percentages were recorded i) for all patients (n=56), ii) for patients undergoing PET/CT (n=33).
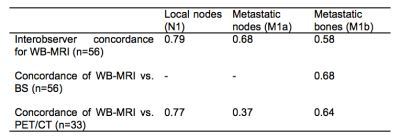
2. The interreader agreement of WB-MRI (n=56) and agreement between WB-MRI and BS (n=56) and PET/CT (n=33) using Kappa (κ).
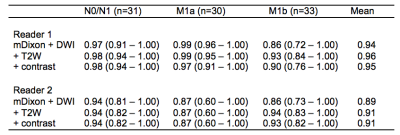
3. ROC-AUC for both WB-MRI readers following each component of the LSR, applying thresholds for each level of suspicion (1 – 6) vs. the reference standard. Youden’s index 5 was used to determine the optimal cut-off of the ROC curve, and thus provide the highest combination of sensitivity and specificity.
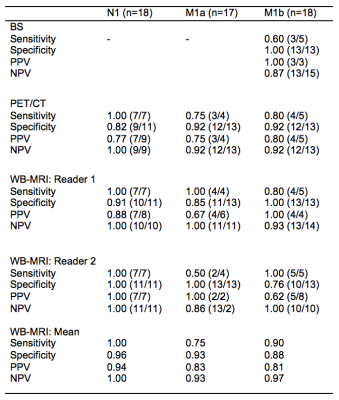
4. The sensitivity, specificity positive (PPV) and negative (NPV) predictive values at each ‘TNM’ stage for the BS, PET/CT and WB-MRI against the reference standard.
RESULTS
The distribution of disease for each staging modality is presented in Figure 2.
Concordance statistics (κ) between WB-MRI readers and modalities are presented in figure 3.
ROC-AUC statistics following each part of the LSR are presented in figure 4.
Youden’s index confirmed the optimal cutoff of the ROC-AUC was ≥4 in all cases. The sensitivity and specificity for BS, 18F-choline-choline PET CT and WB-MRI were therefore calculated using a threshold of ≥4 as positive against the follow-up reference standard. Results are displayed in figure 5.
CONCLUSION
WB-MRI provides high levels of interobserver concordance, intermodality concordance and diagnostic accuracy for both nodal and metastatic bone disease, with higher levels of sensitivity than BS for metastatic disease, and similar performance to PET/CT. T2W and post contrast mDixon have no additive diagnostic value above T1W and DWI alone.Acknowledgements
This research was supported by a grant from the Comprehensive Cancer Imaging Centre (a UCL/KCL collaboration) and Cancer Research UK.References
1. Bjurlin MA, Rosenkrantz AB, Beltran LS, Raad RA, Taneja SS. Imaging and evaluation of patients with high-risk prostate cancer. Nat Rev Urol 2015; 12: 617–28.
2. Hövels a. M, Heesakkers R a M, Adang EM, et al. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: a meta-analysis. Clin Radiol 2008; 63: 387–95.
3. Shen G, Deng H, Hu S, Jia Z. Comparison of choline-PET/CT, MRI, SPECT, and bone scintigraphy in the diagnosis of bone metastases in patients with prostate cancer: a meta-analysis. Skeletal Radiol 2014; : 1503–13.
4. Roach M, Hanks G, Thames H, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: Recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol 2006; 65: 965–74.
5. Youden WJ. INDEX FOR RATING DIAGNOSTIC TESTS. Cancer. 1950 Jan;3(1):32-5.
Figures