2555
Magnetic resonance spectroscopy of localized prostate cancer: assessment of antitumor effects of intra-prostatic hormone deprivation therapy1Department of Medical Physics, Uppsala University Hospital, Uppsala, Sweden, 2Department of Urology, Uppsala University Hospital, Uppsala, Sweden, 3LIDDS AB, Uppsala, Sweden
Synopsis
A novel controlled release formulation based on calcium sulphate as drug carrier loaded with the antiandrogen 2-hydroxiflutamide as the active pharmaceutical agent was injected locally into the prostate in patients with prostate cancer. Single-voxel and 2D MRSI using a surface coil were used to investigate the treatment efficiency. The results demonstrate usefulness of both MRS techniques to detect metabolic atrophy caused by long-term local hormone-deprivation therapy. The presence of metabolic atrophy reflects the antitumor effects of the study drug formulation 6 weeks after the intraprostatic injections.
Introduction
Low or medium grade prostate cancer is diagnosed in a large majority of prostate cancer patients.1 A typical treatment for these men includes various types of testosterone deprivation therapies, often associated with hormonal side effects. A less invasive treatment with fewer side effects (metabolic syndrome, cardiovascular events, sexual dysfunction, etc.) would be a desirable option. One such treatment option is Liproca® Depot, a local intraprostatic hormone deprivation therapy investigated in this work.2 Currently, T2- and diffusion-weighted MRI is commonly used during the follow-up of patients undergoing therapy. However, these techniques are of limited diagnostic value because T2-weighted signal intensity and apparent diffusion coefficients of noncancerous and cancerous tissues decrease following therapy. Proton magnetic resonance spectroscopy (1H-MRS) improves the ability of MRI to monitor the treatment response.3 Spectral intensities of detectable metabolites such as, choline (Cho), polyamines (PA), creatine (Cr), and citrate (Cit) decrease with increasing exposure during therapy. The final step is metabolic atrophy, where signals from metabolites are undetectable. Metabolic atrophy is indicative of successful treatment because the growth of normal and cancer cells cannot occur without metabolism. The aim of this study was to assess usefulness of 1H-MRS for monitoring the treatment efficiency of a novel transrectally injected intraprostatic drug depot formulation with the antiandrogen 2-hydroxyflutamide.Methods
Eleven prostate cancer patients scheduled for prostatectomy participated in this study. Liproca® Depot is composed of 2-hydroxyflutamide (2-HOF) encapsulated in a calcium sulphate drug carrier matrix (NanoZolid® technology, LIDDS, Sweden).2,4 Following intraprostatic injection of Liproca® Depot (containing 30 mg 2-HOF per mL prostate), the depot solidifies in vivo forming a solid depot which dissolves and releases 2-HOF gradually, providing a local drug concentration in the prostate over a prolonged period of time (up to six months). The follow-up time in this study was 6 weeks. MRI, single-voxel MRS and 2D magnetic resonance spectroscopic imaging (2D MRSI) of the prostates were performed before injection and 6 weeks after the injection, just before the prostatectomy. All measurements were performed with a 3 T scanner (Achieva, Philips, Best, The Netherlands) using a whole-body coil for excitation and surface phase-array coil for receiving. Spectroscopic experiments and data processing were described elsewhere.5,6 Therapy results were assessed by quantification of (Cho+PA+Cr)/Cit spectral intensity ratio and by signal-to-noise ratio (SNR) of the spectra.Results
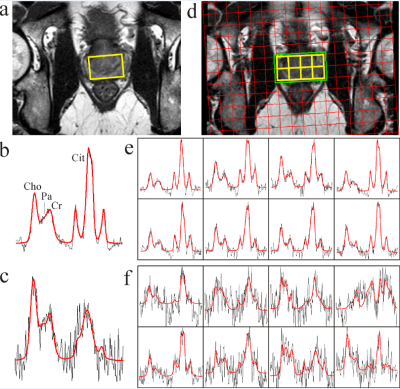
Figure 1 shows single-voxel and 2D MRSI spectra of a representative patient before the drug injection into the prostate and 6 weeks after the treatment. The spectral intensities of Cho, PA, Cr, and Cit decreased (Fig. 1 c and f). Consequently SNR decreased. These features reveal the presence of partial metabolic atrophy that is seen in benign as well as in prostate cancer tissues. Cit concentration decreased faster than Cho and Cr, resulting in an increase of (Cho+PA+Cr)/Cit ratio. From quantitative point of view more reliable single-voxel MRS before and after treatment was possible to evaluate in 5 patients. For these patients, median (Cho+PA+Cr)/Cit ratio increase was 71% (range:12-110%), and median SNR decrease was 44% (range: 22-80%). These finding suggest that local hormone deprivation therapy has not reached its full effect after 6 weeks. Anti-androgen related side effects, typical for oral use of the same drug, were not detected. Frequency of adverse effects were similar to those of transrectal prostate biopsy, i.e. a few % of urinary disorders or infections.Discussion
The study formulation was designed to deliver a higher initial release rate (burst) followed by a slower and prolonged drug release (maintenance). Our results have shown that intra-prostatic injection of Liproca® Depot caused an overall reduction in prostate tissue metabolism interpreted as cellular (metabolic) atrophy. The increase in (Cho+PA+Cr)/Cit spectral intensity ratio and SNR decrease after the treatment reveal an antitumor treatment effect.Conclusion
This study support the use of both single-voxel MRS and MRSI to detect metabolic atrophy in patients with locally advanced prostate cancer treated with long-term hormone-deprivation therapy. The detected metabolic atrophy confirmed an antitumor effect of the study formulation.Acknowledgements
No acknowledgement found.References
1. Sieh W, Lichtensztajn DY, Nelson DO, et al. Treatment and mortality in men with localized prostate cancer: A population-based study in California. Open Prostate Cancer J. 2013;6:1-9.
2. Ahlström H, Tammela T, Häggman M, et al. An intraprostatic modified release formulation with 2-hydroxyflutamide for patients with localized prostate cancer. J Urology. 2017, in press.
3. Mueller-Lisse UG, Swanson MG, Vigneron DB, et al. Magnetic resonance spectroscopy in patients with locally confined prostate cancer: association of prostatic citrate and metabolic atrophy with time on hormone deprivation therapy. Eur Radiol. 2007;17:371-378.
4. Thomas MV, Puleo DA. Calcium sulfate: Properties and clinical applications. J Biomed Mat Res. 2009;88(2):597-610.
5. Weis J, Jorulf H, Bergman A, et al. MR spectroscopy of the human prostate using surface coil at 3 T: metabolite ratios, age-dependent effects, and diagnostic possibilities. J Magn Reson Imag. 2011;34:1277-1284.
6. Weis J, von Below C, Tolf A, et al. Quantification of metabolite concentrations in benign and malignant prostate tissues using 3D proton MR spectroscopic imaging. J Magn Reson Imag. 2017;45:1232-1240.
Figures