Shirin Sabouri1, Silvia D. Chang2, Edward C. Jones3, S. Larry Goldenberg 4, Peter C. Black4, and Piotr Kozlowski2
1Physics and Astronomy, University of British Columbia, Vancouver, BC, Canada, 2Radiology, University of British Columbia, Vancouver, BC, Canada, 3Pathology and Laboratory Medicine, University of British Columbia, Vancouver, BC, Canada, 4Urologic Sciences, University of British Columbia, Vancouver, BC, Canada
Synopsis
T2W imaging is an
important sequence in the PIRADSv2 guideline for scoring prostatic lesions. The
apparent contrast between malignant and non-malignant tissues on T2W images depends
on the time of echo (TE). In this study we have investigated the effect of TE
on the contrast-to-noise ratio (CNR) between malignant and non-malignant
tissues. We have acquired and analyzed T2W data from 12
patients. Our results show that
CNR increases abruptly for TEs between 25 and 175ms. After CNR reaches its
maximum at 175ms it gradually decreases.
Our findings may be used toward improvement of T2W protocols for diagnosis of
prostatic carcinoma.
Purpose:
To investigate the influence
of time of echo in T2 weighted (T2W) magnetic resonance imaging (MRI) on the
apparent contrast-to-noise ratio (CNR) between malignant and non-malignant prostate
tissue. Introduction:
T2W sequence is part of the prostate imaging and
data reporting system version2 (PIRADSv2). In general, clinically significant
cancers usually appear as hypointense lesions on T2W images. As the T2W signal
is echo time dependent, the apparent contrast between a tumour and the
neighbouring non-malignant tissue may be different among images acquired at
different effective times of echo (TE). To evaluate the perception of the
distinct differences between tumours and non-malignant tissues on images, it is
useful to measure the corresponding contrast-to-noise ratio (CNR). CNR is
defined as the difference of signal intensity between two regions, scaled to
image noise. This work has been conducted to investigate the influence of TE of
T2W images on the measured CNR between tumours and non-malignant tissues. We
were particularly interested to determine whether there is a TE value at which
the CNR is maximum.Methods:
12 patients with biopsy
proven prostatic carcinoma (PCa) were examined at a 3T scanner prior to
undergoing radical prostatectomy. MR signals were acquired with combined
endorectal/pelvic phased-array coils. A 3D multi-echo GRASE sequence
(TR/TE=3061/25ms,NE=64,FOV=240×240×40mm3,voxel-size=1×1×4mm3,matrix-size=240×240) was used to obtain T2W images at different TEs. In total,
30 slices that contained both non-malignant tissue and tumours with cross-sectional area≥100mm2 were selected for data analysis. Average
values of signal intensity were calculated within tumour and non-malignant
regions-of-interest (ROIs) manually outlined on digitized whole-mount histology
sections registered to MR images. The ROIs were only selected from
peripheral-zone (PZ) due to limited number of transition-zone (TZ) tumours among
the acquired data set. Standard deviation of noise was calculated from ROIs manually
outlined in the area outside the body(air). For each T2W image, the CNR was
calculated by subtracting the average signal intensity within tumour ROI from
the average signal intensity within non-malignant ROI and dividing the result
by the standard deviation of noise. Curves of CNR as a function of TE were
generated for each individual slice. The individual curves were then added and
averaged to generate the curve of average CNR as a function of TE. Results:
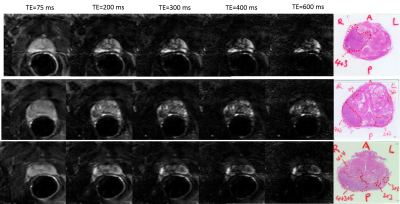
Representative T2W images and the corresponding
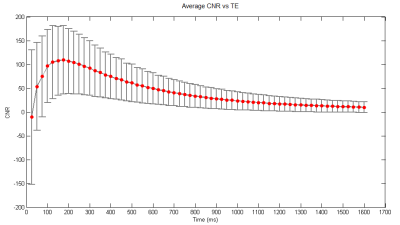
histology sections are presented in Figure1. The graph of average CNR versus TE
is presented in Figure2. Our results demonstrate that CNR increases
abruptly from -10 to 110 for TEs between 25 and 175ms. The maximum CNR occurs
at TE=175ms. After reaching its peak
value, CNR gradually decreases to a minimum of 10 at 1600ms.Discussion & Conclusions:
In this study, we
investigated the influence of TE in T2W on the CNR between malignant and
non-malignant tissues. Our results must be interpreted with caution as the
analyzed data were sampled from a limited range of tumour grades (Gleason
pattern≥4+3).
Although we can expect a similar rise-fall pattern in CNR for other tumour
grades, the location of the peak might be different for different grades.
Therefore, when interpreting our results, we focus on the variation pattern of
CNR as a function of TE, rather than the location of its peak value. To
optimize the CNR for all tumour grades, a study involving a larger number of
patients and broader range of Gleason scores is needed.
Another limitation
of this study is that its data and findings lend themselves only to PZ, for
which T2W is not the dominant sequence in the PIRADSv2, but still helpful in
providing overall score, particularly for caveats. Performing similar analyses in
TZ could lead to a more significant contribution, as T2W is the dominant
sequence for scoring TZ lesions in PIRADSv2. To expand this study to TZ, more
patient data should be included, because the prevalence of tumours in TZ is much
less compared to PZ. Another limitation to be noted is that in this study noise
calculation was performed using a simplistic approach. As our imaging involved
sensitivity encoding, the noise had spatial dependence and a more accurate
estimation of noise could have been performed by considering this effect. Such complex
estimation was not implemented in this study; however, more accurate noise
estimate will result in a different scale factor on the CNR vs TE graph, and would
likely not affect the overall findings of this study. In conclusion, in this
work we characterized the dependence of CNR on the TE of T2W imaging, and we
showed that there is a TE that maximises the CNR. This result may have
important implications in clinical settings as acquiring T2W images with
optimal TE may provide a significant improvement in the detectability of
tumours and improve the sensitivity of diagnosis of PCa.Acknowledgements
This
study has been supported by the Canadian Institutes of Health Research. References
1. PI-RADS.
Prostate Imaging Reporting and Data System, Version2. American College of
Radiology Website. 2015. Retrieved on 19/04/2017 from http:/ ̴ /www.acr.org//media/ACR/Documents/PDF/QualitySafety/Resources/PIRADS/PIRADS%20V2.pdf