2551
Magnetisation transfer in human liver and kidney through acquisition of the z-spectrum1Sir Peter Mansfield Imaging Centre, University of Nottingham, Nottingham, United Kingdom, 2National Institute for Health Research (NIHR) Nottingham Biomedical Research Centre, Nottingham, United Kingdom
Synopsis
This study explores the feasibility of measuring magnetisation transfer in both the liver and kidney through acquisition of a full z-spectrum. In this study we developed a protocol to reduce artefacts from respiration and blood flow pulsatility and measured relative amounts of MT in both the liver and kidney medulla by modelling MT with a super-Lorentzian lineshape and fitting to the acquired spectrum. This will be relevant in monitoring fibrosis in abdominal organs.
Purpose
Recent studies have shown that magnetisation transfer ratio (MTR) imaging can be used to identify intestinal fibrosis1,2, and similarly renal fibrosis has been associated with MTR changes in mice and pigs3,4. To date there has only been one study of MTR in the human liver5, and a similar study has been performed in the kidney6. However MTR is only a qualitative measure, whereas z-spectrum imaging offers the possibility of quantifying MT pool size by correcting for B0 and B1 errors. Furthermore the z-spectrum may allow other features to be identified, particularly related to liver or renal fibrosis3. This work expands on previous work by overcoming some of the challenges involved in acquiring full abdominal z-spectrum data from the liver and kidney.Aim
To develop a method of quantifying MT in the liver and kidney at 3T, overcoming the effects of respiration and blood flow.Methods
2 healthy subjects (1M, 1F, age=23) were scanned using a Philips Ingenia 3.0T system. Z spectra were acquired using an MT-TFE sequence7 (B1=2.19μT, single slice, 30 Gaussian-windowed sinc pulses, pulse duration/spacing = 30/80ms, single-shot, TFE readout). Initial investigations revealed that the signal was strongly dependent on both the respiratory and cardiac cycle, and so the sequence was adjusted to include respiratory and cardiac triggering. An axial-oblique slice was acquired in which both the liver and kidney tissue were visible. 47 frequencies were acquired between ±50,000Hz (±391ppm) in order to sample the full width of the MT peak8, with acquisition of all 47 frequencies taking 2min30s. Z-spectrum acquisition was repeated 5 times so that any anomalies caused by mistimed triggering could be removed.
The liver and kidney medulla were masked to exclude blood vessels, and subsequently the highest and lowest signals recorded at each off-resonance frequency were discarded to reduce scatter. The remaining points were averaged and two peaks were fitted to the resulting spectra: a narrow Lorentzian representing the water peak, and a wide super-Lorentzian representing the MT peak9, with the centre position of this peak allowed to vary. The amplitude and position of this peak was recorded after fitting for the lowest sum of squares difference, the amplitude being proportional to the size of the bound pool (M0,b) (neglecting T1 effects). Error bounds were estimated from the 5 recorded spectra for each subject by fitting the z-spectra seperately.
Results
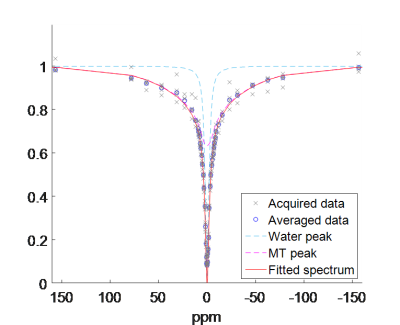
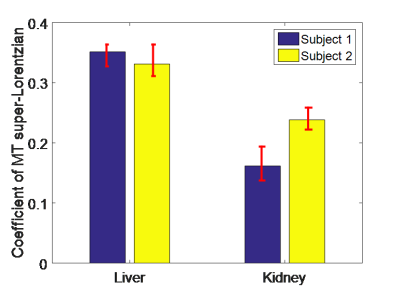
Figure 1 shows the z-spectrum collected from the liver of one subject, including the individual and averaged acquired spectra, the final fit, and the individual peaks corresponding to free water and MT. This gives an indication of the noise that had to be accounted for (note that the points furthest above and below the median value for each dynamic were excluded from the fit). No CEST or NOE effects could be clearly identified. The results of the least sum of squares fit revealed that the MT super-Lorentzian was centred at -0.25ppm relative to water. The MT pool sizes are displayed in Figure 2 and show a larger MT effect in the liver than the kidney medulla.Discussion
Figure 1 shows that blood flow and irregular respiration can cause significant noise in the z-spectrum, however performing multiple repeats in a reasonable imaging time allowed us to identify outliers and to plot a smooth spectrum. Figure 2 shows measured MT values in healthy subjects. Acquisition of the full z-spectrum allows a reduction in systematic errors due to B0 and B1 inhomogeneities. Furthermore MT could be quantified with AREX10 or fully quantified using a look up table11 if a T1 map were also acquired. Given the short scan time and the respiratory triggered acquisition, further repeats could be acquired to increase the signal to noise ration in the z-spectrum with minimal patient discomfort. This technique has the potential to identify fibrotic changes in the liver and kidney. In addition, the short scan time for a single spectrum means that this method could be translated to study fibrosis in the gut wall, when combined with an antispasmodic drug, which immobilises the gut wall for around 5-10 minutes. The methods developed here will be translated to 7T to allow the study of CEST in the abdomen.Conclusion
MT pool size can be quantified in the liver and kidney at 3T from the z-spectrum acquired in a short scan time. The MT peak is symmetric around the water peak.Acknowledgements
Andrew Carradus holds a studentship with the Haydn Green Foundation.
This work was supported by the National Institute for Health Research (NIHR) Nottingham Biomedical Research Centre.
References
1. Pazahr, Shila, et al. "Magnetization transfer for the assessment of bowel fibrosis in patients with Crohn’s disease: initial experience." Magnetic Resonance Materials in Physics, Biology and Medicine 26.3 (2013): 291-301.
2. Adler, Jeremy, et al. "Magnetization transfer helps detect intestinal fibrosis in an animal model of Crohn disease." Radiology 259.1 (2011): 127-135.
3. Jiang, Kai, et al. "Noninvasive Assessment of Renal Fibrosis with Magnetization Transfer MR Imaging: Validation and Evaluation in Murine Renal Artery Stenosis." Radiology 283.1 (2016): 77-86.
4. Jiang, Kai, et al. "Magnetization Transfer Magnetic Resonance Imaging Noninvasively Detects Renal Fibrosis in Swine Atherosclerotic Renal Artery Stenosis at 3.0 T." Investigative Radiology 52.11 (2017): 686-692.
5. Alanen, A., et al. "MR and magnetisation transfer imaging in cirrhotic and fatty livers." Acta Radiologica 39.4 (1998): 434-439.
6. Ito, Katsuyoshi, et al. "Magnetisation transfer MR imaging of the kidney: evaluation at 3.0 T in association with renal function." European radiology 23.8 (2013): 2315-2319.
7. Mougin, Olivier, et al. "High‐resolution imaging of magnetisation transfer and nuclear Overhauser effect in the human visual cortex at 7 T." NMR in Biomedicine 26.11 (2013): 1508-1517.
8. Wolff, Steven D., and Robert S. Balaban. "Magnetization transfer contrast (MTC) and tissue water proton relaxation in vivo." Magnetic resonance in medicine 10.1 (1989): 135-144.
9. Morrison, Clare, and R. Mark Henkelman. "A model for magnetization transfer in tissues." Magnetic resonance in medicine 33.4 (1995): 475-482.
10. Zaiss, Moritz, et al. "Inverse Z‐spectrum analysis for spillover‐, MT‐, and T1‐corrected steady‐state pulsed CEST‐MRI–application to pH‐weighted MRI of acute stroke." NMR in biomedicine 27.3 (2014): 240-252.
11. Geades, Nicolas, et al. "Quantitative analysis of the z‐spectrum using a numerically simulated look‐up table: Application to the healthy human brain at 7T." Magnetic resonance in medicine 78.2 (2017): 645-655.
Figures