2544
The Effects of Helical Flow Patterns, Confluence Angle, and Flow Distribution in the Portal Vein1Mechanical Engineering, University of Wisconsin-Madison, Madison, WI, United States, 2Radiology, University of Wisconsin-Madison, Madison, WI, United States, 3Medical Physics, University of Wisconsin-Madison, Madison, WI, United States, 4Biomedical Engineering, University of Wisconsin-Madison, Madison, WI, United States, 5Medicine, University of Wisconsin-Madison, Madison, WI, United States, 6Emergency Medicine, University of Wisconsin-Madison, Madison, WI, United States
Synopsis
The hemodynamics of the liver in normal and diseased conditions are not fully understood. In this study, 4D flow MRI and computational modeling were used to analyze the effects of portal venous flow patterns at the spleno-mesenteric confluence. Specifically, the geometric configuration of the confluence on intra-hepatic portal circulation in healthy subjects and cirrhotic patients before and after a meal challenge was analyzed. Significant correlations between flow distribution, helicity, geometry, and flow patterns were observed, and differences between normal and pathological flow were also characterized.
Introduction
Liver disease can have heterogeneous lobar distribution, which may be a result of imbalanced blood flow distribution between portal venous branches. The hepatic blood flow magnitude and distribution can be characterized to provide insight into the pathophysiology of liver disease that affects or depends on the hepatic vasculature.[1],[2] However, the underlying patterns and dynamics of hepatic blood flow have not yet been comprehensively studied.
One particular pattern that has been observed in the hepatic portal vein (PV) is a helical flow structure that develops distal to the confluence of the splenic vein (SV) and superior mesenteric vein (SMV).[3] The cause and downstream effects of this portal helix flow pattern on the surrounding vasculature in states of health and disease are currently not well understood. Emerging imaging and computational methods to visualize and quantify blood flow, however, now make the evaluation of flow patterns at the spleno-mesenteric confluence (SMC) feasible.[4],[5],[6]
Thus, the purpose of this study was to use 4D flow MRI and computational simulation to examine the effects of the portal helix flow pattern and SMC geometry on intra-hepatic portal hemodynamics.
Methods
In this IRB-approved and HIPAA-compliant study, 12 subjects (6 healthy, 6 cirrhotic) were imaged before and after a meal challenge[2] on a clinical 3T MRI system (Discovery MR 750, GE Healthcare, Waukesha, WI) with a 32-chanel body coil (NeoCoil, Pewaukee, WI). 4D velocity mapping was performed using a cardiac-gated time-resolved 3D radially undersampled phase contrast (PC) acquisition (5-point PC-VIPR) with increased velocity sensitivity performance. [7] Acquisition and reconstruction parameters were previously described.[2],[6]
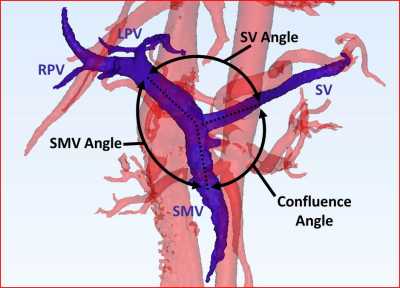
3D models, segmented from PC angiograms using MIMICS (Materialise, Leuven, Belgium), were exported to 3-matic (Materialise, Leuven, Belgium) to measure the angle of the SMC confluence (Figure 1).
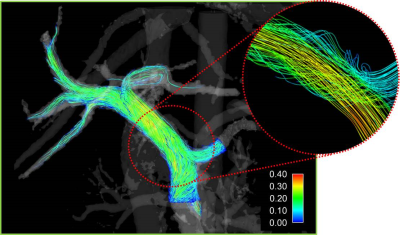
Segmented models, along with the 4D flow data were exported to Ensight (CEI, Apex, NC). Cut-planes were placed in the splenic vein (SV), superior mesenteric vein (SMV), and the left (LPV) and right (RPV) portal veins, where flow measurements were made. Velocity streamlines were generated from the SV and SMV planes, and tracked throughout the portal vein (Figure 2). The number of streamlines exiting the LPV and RPV were quantified to represent right and left liver lobe flows. Helicity, vorticity, and kinetic energy were also quantified throughout the portal anatomy.
In some cirrhotic patient cases, limited SNR performance inhibited reliable quantification and visualization of flow distribution in distal portions of the portal vein. Therefore, MRI based computational simulation [6] was used to augment distal portal vein flow distribution analysis in these cases. 4D flow MRI data were then compared across pre/post meal challenge and healthy/cirrhotic subject categories using a Student’s paired t-test and linear regression for correlation analysis.
Results
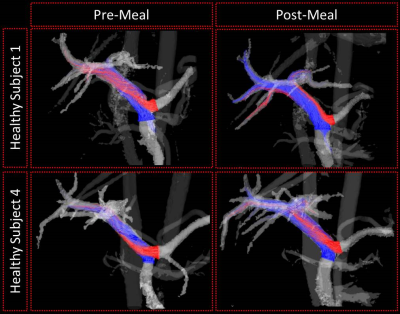
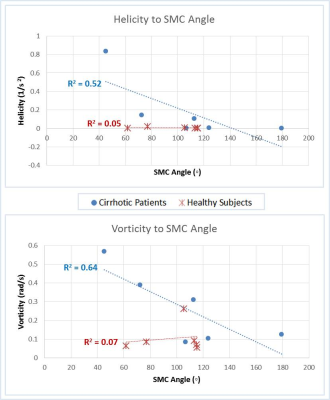
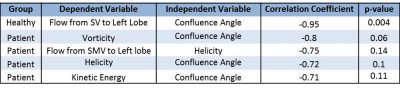
Correlation relationships between measured hemodynamic metrics are summarized in Table 1, and significant results are described as follows. In fasting healthy subjects, the proportion of flow from the SV to the left lobe of the liver decreased with increasing confluence angle (Figure 1). However, this trend changed in healthy subjects after a meal (Figure 3). Further, these trends were not present in the cirrhotic patients. Vorticity, helicity, and kinetic energy all decreased with increasing confluence angle in patients, but not in healthy subjects. Helicity and vorticity were non-significantly higher in patients than in healthy subjects (p=0.12, p=0.09) (Figure 4).Discussion
Liver hemodynamics are not fully understood. To examine the effects of the SMC geometry on blood flow in the portal venous system, we studied six healthy subjects and 6 patients with cirrhosis, both before and after an induced liver hemodynamic stress (meal challenge). We found that the geometry of the SMC significantly affected the portal flow distribution between liver lobes in these healthy subjects before stress is induced. However, this was not the case for patients with cirrhosis. Additionally, we observed greater helical flow and more flow distribution dependence on helix formation in patients with cirrhosis than was observed in healthy subjects. Finally, we demonstrated the use of computational simulation to study intricate flow patterns that are difficult to observe with imaging alone when regional SNR performance is limited. The methods described in this work will be further developed with the intent of improving characterization of liver disease and for surgical planning.[6]Conclusion
Using a combination of 4D flow MRI and computational simulations, we found significant correlations between portal vessel geometry, flow structure development, and blood flow distribution in healthy and cirrhotic subjects, as well as differences in these parameters before and after a meal challenge. With further development, the methods of this study may be used for clinical analysis and planning tools.Acknowledgements
The research presented was supported by the NIH (UL1TR000427, TL1TR000429, K24 DK102595 and R01 DK096169). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors also wish to acknowledge support from GE Healthcare who provides research support to the University of Wisconsin.References
[1] Lara, M., Chen, C. Y., Mannor, P., Dur, O., Menon, P. G., Yoganathan, A. P., and Pekkan, K., 2011, "Hemodynamics of the hepatic venous three-vessel confluences using particle image velocimetry," Ann Biomed Eng, 39(9), pp. 2398-2416.
[2] Roldán-Alzate, A., Frydrychowicz, A., Said, A., Johnson, K. M., Francois, C. J., Wieben, O., and Reeder, S. B., 2015, "Impaired regulation of portal venous flow in response to a meal challenge as quantified by 4D flow MRI," J Magn Reson Imaging, 42(4), pp. 1009-1017.
[3] Van As, A. B., Hickman, R., Engelbrecht, G. H., Makan, P., Duminy, F., and Kahn, D., 2001, "Significance of the portal vein helix," S Afr J Surg, 39(2), pp. 50-52.
[4] Roldán-Alzate, A., Frydrychowicz, A., Niespodzany, E., Landgraf, B. R., Johnson, K. M., Wieben, O., and Reeder, S. B., 2013, "In vivo validation of 4D flow MRI for assessing the hemodynamics of portal hypertension," J Magn Reson Imaging, 37(5), pp. 1100-1108.
[5] Rosenthal, S. J., Harrison, L. A., Baxter, K. G., Wetzel, L. H., Cox, G. G., and Batnitzky, S., 1995, "Doppler US of helical flow in the portal vein," Radiographics, 15(5), pp. 1103-1111.
[6] Rutkowski, D. R., Reeder, S. B., Fernandez, L. A., and Roldán-Alzate, A., 2017, "Surgical planning for living donor liver transplant using 4D flow MRI, computational fluid dynamics and in vitro experiments," Computer Methods in Biomechanics and Biomedical Engineering: Imaging & Visualization, pp. 1-11.
[7] Johnson, K. M., Lum, D. P., Turski, P. A., Block, W. F., Mistretta, C. A., and Wieben, O., 2008, "Improved 3D phase contrast MRI with off-resonance corrected dual echo VIPR," Magn Reson Med, 60(6), pp. 1329-1336.
Figures