2542
Liver Imaging Reporting and Data System Category 5: 3.0 T MR Predictors of Microvascular Invasion and Early Recurrence after Hepatectomy for Hepatocellular Carcinoma1Department of Radiology, the Third Affiliated Hospital of Sun Yat-sen University(SYSU), Guangzhou, China, 2Department of Radiology, University of California San Diego, San Diego, CA, Armenia
Synopsis
Hepatocellular carcinoma (HCC) is the fifth most common malignancy worldwide. Tumor microvascular invasion (MVI) predicts early posthepatectomy HCC recurrence, but usually cannot be determined until the tumor is surgically removed and analyzed histologically. The capability preoperatively to predict MVI and early postsurgical recurrence would represent an advance by informing optimal selection of surgical candidates. Here we show that in combination with a AFP (a tumor biomarker), two Liver Imaging Reporting and Data System (LI-RADS) imaging features (mosaic architecture, corona enhancement) can predict MVI and three features (tumor number, mosaic architecture, absence of intralesional fat) can predict early recurrence.
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common malignancy worldwide 1, 2. Tumor microvascular invasion (MVI) is a well-known major prognostic factor and predicts early posthepatectomy HCC recurrence 3, but usually cannot be determined until the tumor is surgically removed and analyzed histologically. The capability preoperatively to predict MVI and early postsurgical recurrence would represent an advance by informing optimal selection of surgical candidates. It has been reported that tumor signal intensity, enhancement pattern, and ADC values on MR imaging may predict MVI preoperatively 3-7. However, assessment of these features is not standardized. A more standardized assessment is needed. The Liver Imaging Reporting and Data System (LI-RADS) provides standardized terminology, interpretation, and reporting of imaging examinations for the diagnosis of HCC 8. Recently, it was reported that LI-RADS features may also have prognostic implications 9. The purpose of this study was to investigate whether LI-RADS v2014 magnetic resonance imaging (MRI) features in combination with alpha fetoprotein (AFP) could predict MVI and early posthepatectomy recurrence.Methods
Our institutional review board approved this retrospective cohort study and waived informed consent requirement. From March 2014 to July 2017, 180 patients (160 men and 20 women) underwent hepatectomy for HCC and had a preoperative 3.0 T dual-arterial dynamic contrast-enhanced MRI examination within one month preoperatively. Major and ancillary features of the LR-5 observations were assessed indepedendently according to LI-RADS v2014 by two abdominal radiologists with 5 years and 24 years of experience in liver MRI, respectively, unaware of clinical, laboratory, pathologic, and follow-up information. The largest LR-5 observation was assessed in the 32 patients with more than one HCC nodule. Disagreements were resolved by consensus. HCCs were diagnosed and MVI was assessed by a liver pathologist with 21 years of experience on hematoxylin-and-eosin-stained slides. Prehepatectomy AFP levels were recorded. Patients were followed every 3 months for the first year posthepatectomy and every 6 months thereafter. Early recurrence was defined as a new HCC tumor with typical imaging features and elevated AFP within 1 year after hepatectomy. The time to recurrence was defined as the number of days from hepatectomy until recurrence (if any) was first diagnosed. Logistic regression was performed to assess MRI features for predicting MVI. Variables with P˂0.1 in univariate logistic regression analysis were applied to a multivariate logistic regression analysis. Kaplan–Meier recurrence curves were generated. The frequency of early recurrence was compared among subgroups using log-rank analysis. A Cox proportional hazards model was used for univariate and multivariate analyses of factors related to recurrence.Results
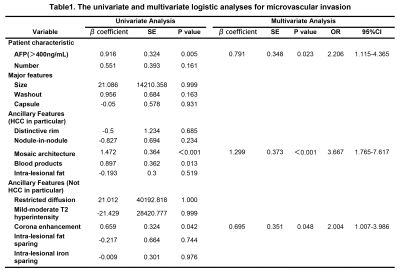
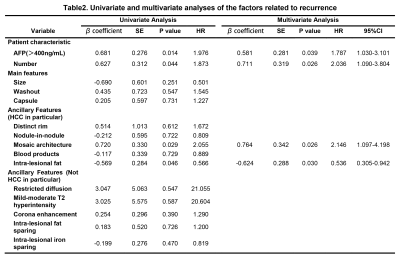
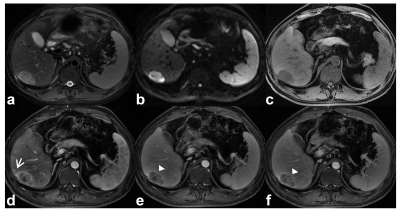
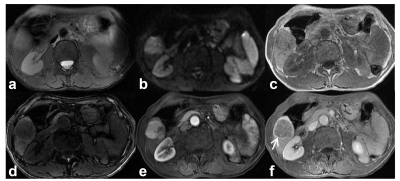
Among the 180 analyzed HCC tumors, average size was 49 mm. 81 tumors had MVI. Mosaic architecture (β=1.299, P˂0.001), corona enhancement (β=0.695, P=0.048), and AFP>400ng/mL (β=0.791, P=0.023) were independent predictors of MVI (Table.1, figure.1). Tumor number (HR=2.036, P=0.026), mosaic architecture (HR=2.146, P=0.026), absence of intralesional fat (HR=1.87, P=0.030), and AFP>400ng/mL (HR=1.787, P=0.039) were independent predictors of early posthepatectomy recurrence (Table.2, figure.2).Discussion
HCC is the only tumor that can be diagnosed noninvasively by imaging-based criteria without confirmatory biopsy 10-12. Our study suggests that in combination with a AFP (a tumor biomarker), two LI-RADS MRI features (mosaic architecture, corona enhancement) can predict MVI and three LI-RADS MRI features (tumor number, mosaic architecture, absence of intralesional fat) can predict early recurrence. If validated by future independent studies, these LI-RADS MRI features could be used in combination with AFP preoperatively to predict MVI and early posthepatcomy recurrence, information which may help inform optimal surgical decision making. Limitations of our study were that it was single-center and retrospective, and it focused only on 3.0 T MRI.Conclusion
Our preliminary results suggest that in combination with AFP>400ng/mL, mosaic architecture and corona enhancement were independent predictors of MVI and tumor number, mosaic architecture, and absence of intralesional fat were independent predictors of early posthepatecomy recurrence. Independent validation of our results is needed, ideally in a prospective multicenter study.Acknowledgements
noReferences
1. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA: A Cancer Journal for Clinicians 2015;65(2):87-108.
2. Bertuccio P, Turati F, Carioli G, et al. Global trends and predictions in hepatocellular carcinoma mortality. J Hepatol 2017;67(2):302-309.
3. Lee S, Kim SH, Lee JE, et al. Preoperative gadoxetic acid-enhanced MRI for predicting microvascular invasion in patients with single hepatocellular carcinoma. J Hepatol 2017;67(3):526-534.
4. Renzulli M, Brocchi S, Cucchetti A, et al. Can Current Preoperative Imaging Be Used to Detect Microvascular Invasion of Hepatocellular Carcinoma? Radiology 2016;279(2):432-442.
5. Kim M, Lee M, Choi J, et al. Imaging features of small hepatocellular carcinomas with microvascular invasion on gadoxetic acid-enhanced MR imaging. European Journal of Radiology 2012;81(10):2507-2512.
6. An C, Kim DW, Park YN, et al. Single Hepatocellular Carcinoma: Preoperative MR Imaging to Predict Early Recurrence after Curative Resection. Radiology 2015;276(2):433-443.
7. Xu P, Zeng M, Liu K, et al. Microvascular invasion in small hepatocellular carcinoma: is it predictable with preoperative diffusion-weighted imaging? J Gastroenterol Hepatol 2014;29(2):330-336.
8. Mitchell DG, Bruix J, Sherman M, et al. LI-RADS (Liver Imaging Reporting and Data System): summary, discussion, and consensus of the LI-RADS Management Working Group and future directions. Hepatology 2015;61(3):1056-1065.
9. An C, Park S, Chung YE, et al. Curative Resection of Single Primary Hepatic Malignancy: Liver Imaging Reporting and Data System Category LR-M Portends a Worse Prognosis. AJR Am J Roentgenol 2017;209(3):576-583.
10. European Association for Study of Liver, European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56(4):908-943.
11. Bruix J, Sherman M. Management of hepatocellular carcinoma: An update. Hepatology 2011;53(3):1020-1022.
12. Tang A, Cruite I, Mitchell DG, et al. Hepatocellular carcinoma imaging systems: why they exist, how they have evolved, and how they differ. Abdominal Radiology 2017. doi: 10.1007/s00261-017-1292-3. [Epub ahead of print]
Figures