2531
MR Imaging Perfusion and Diffusion analysis to assess preoperative Short Course Radiotherapy response in locally advanced rectal cancer: Standardized Index of Shape by DCE-MRI and Intravoxel Incoherent Motion derived parameters by DW-MRI1National Cancer Institute of Naples Pascale Foundation, Naples, Italy
Synopsis
Aim of this study is to determine the diagnostic performance of MR imaging for the assessment of tumor response after Short Course Radiotherapy (SCR) in patients with LARC using Standardized Index of Shape (SIS) obtained by DCE-MRI and using ADC, DKI and IVIM derived parameters obtained by DW-MRI.
We demostrated that SIS is a hopeful DCE-MRI angiogenic biomarker to assess preoperative treatment response after SCR with delayed surgery and it permits to discriminate pCR allowing to direct surgery for tailored and conservative treatment.
Introduction
Short Course Radiotherapy (SCR) is known to be a valuable therapeutic option in patients with locally advanced rectal cancer (LARC). A recent meta-analysis [4] reported that SCR with immediate surgery is as effective as long CRT with deferred surgery in terms of overall and disease-free survival rates, local and distant control, and toxicity. Also, SCR with Delayed Surgery (SCRDS) (after 4~8 weeks), optional therapy described for patients with locally advanced tumours who are not fit for CRT, leads to similar results in terms of negative margin resection percentage and satisfactory results about the downstaging and pathological response rate compared to traditional preoperative CRT [5-13]. The use of new imaging modalities to make individual assessments of therapy response could be of great clinical value to adjust subsequent strategies tailored for each patient. Such strategies range from a tailored surgical approach, to administering an adjuvant regimen, or even to a wait and see policy without surgery for patients with high surgical risks [14-16]. Dynamic contrast-enhanced MRI (DCE-MRI) has demonstrated promising to detect residual tumor after pre-surgery CRT [17-21]. Previous studies have been investigated functional parameters derived by DCE-MRI data in rectal cancer [18-21] such as the Standardized Index of Shape (SIS) proposed by Petrillo et al [18] as a simple semi-quantitative parameter capable to differentiate pathological significant and complete response after CRT in LARC. Moreover, in various oncology fields, researchers have recommended the use of diffusion-weighted imaging (DW-MRI) to assess treatment response [22-29]. DW-MRI provides functional information on the tissues microstructure by means of the evaluation of water proton mobility differences [22-23]. By quantifying these differences by means of the individual apparent diffusion coefficient (ADC) it’s possible to quantify biological tumor changes and to monitor treatment response [24-25]. Moreover, using a by-exponential model to analyse DWI-MRI, information both on diffusion and perfusion tissue proprieties derived from Intravoxel Incoherent motion method (IVIM) can be obtained: the pure tissue coefficient (Dt) that describe water macroscopic motion in the cellular interstitial space, the pseudo-diffusion coefficient (Dp) that describe blood microscopic motion in the vessels and the perfusion fraction (fp) that describe the proportion of two different motions [26-29]. Moreover, the conventional DWI model is based on the assumption that water diffusion within a voxel has a single component and follows a Gaussian behavior that water molecules diffuse without any restriction. However, due to the presence of microstructures (i.e., two tissue types or components within one voxel, and organelles and cell membranes), random motion or diffusion of thermally agitated water molecules within biologic tissues exhibits a non-Gaussian phenomena. Jensen and co-workers in 2005 proposed a non-Gaussian diffusion model called as diffusion kurtosis imaging [30]. This model calculates the kurtosis coefficient (K) that signifies the deviation of tissue diffusion from a Gaussian model, and the diffusion coefficient (D) with the correction of non-Gaussian bias. DKI performed better than conventional ADC in tumor detecting and grading. Aim of this study is to determine the diagnostic performance of MR imaging for the assessment of tumor response after SCRDS in patients with LARC using SIS by DCE-MRI and using ADC, DKI and IVIM derived parameters by DW-MRI.
Methods
35 patients with LARC underwent MR scan before and after SCR followed by delayed surgery, retrospectively, were enrolled. SIS, ADC, K, D, Dt, Dp, fp were extracted by MRI for each patient before and after SCR. Tumor regression grade (TRG) were estimated.
The parameters of conventional DWI (ADC), of IVIM (fp, Dt, Dp) and of DKI (MK and MD) were obtained from the multi-b DWI data with all measured b values using the prototype post-processing software Body Diffusion Toolbox (Siemens Healthcare GmbH, Erlangen, Germany).
Receiver operating characteristic curve (ROC), linear classification were performed using the Statistic Toolbox of Matlab R2007a (The Math-Works Inc., Natick, MA).
Results
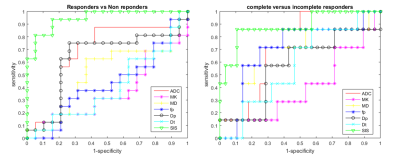
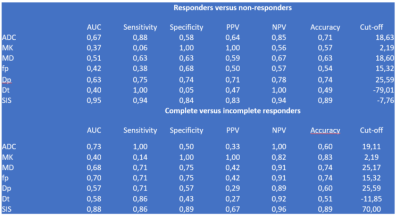
Sixteen patients were classified as responders (TRG≤2) and 19 as non-responders. Seven patients had TRG1 (pathological complete response pCR). The best parameter to discriminate responders by non-responders was SIS (sensitivity 94%, specificity 84%, accuracy 89%, cut-off value=-7.8%). For DWI derived parameters the best results to discriminate responders versus non-responders were obtained with Dp (sensitivity 75%, specificity 74%, accuracy 74%, cut-off value= 25.59%). SIS obtained the best diagnostic performance also to discriminate pCR (sensitivity 86%, specificity 89%, accuracy 89%, cut-off value=68.2%). For DWI derived parameters the best results to detect complete pathological responders were obtained with MK (sensitivity 100%, specificity 100%, accuracy 783%, cut-off value= 2.19%). These results were comparable with those of SIS.Discussion and Conclusion
SIS is a hopeful DCE-MRI
angiogenic biomarker to assess preoperative treatment response after SCR with
delayed surgery and it permits to discriminate pCR allowing to direct surgery
for tailored and conservative treatment. Acknowledgements
Acknowledgement at Robert Grimm (Robert.grimm@siemens.com) and Berthold Kiefer (Berthold.kiefer@siemens.com) for development of the MR Body Diffusion Toolbox, a post-processing software to calculate IVIM and Kurtosis mapsReferences
- Avallone A, Aloj L, Delrio P, Pecori B, Leone A, Tatangelo F, Perri F, Petrillo A, Scott N, Budillon A. Multidisciplinary approach to rectal cancer: are we ready for selective treatment strategies? Anticancer Agents Med Chem. 2013 Jul 1;13(6):852-60.
- Avallone A, Delrio P, Guida C, Tatangelo F, Petrillo A, Marone P, Cascini LG, Morrica B, Lastoria S, Parisi V, Budillon A, Comella P. Biweekly oxaliplatin,raltitrexed, 5-fluorouracil and folinic acid combination chemotherapy during preoperative radiation therapy for locally advanced rectal cancer: a phase I-II study. Br J Cancer. 2006 Jun 19;94(12):1809-15. Epub 2006 May 30.
- Delrio P, Avallone A, Guida C, Lastoria S, Tatangelo F, Cascini GM, Marone P, Petrillo A, Budillon A, Di Marzo M, Palaia R, Albino V, De Rosa V, Parisi V. Multidisciplinary approach to locally advanced rectal cancer: results of a single institution trial. Suppl Tumori. 2005 May-Jun;4(3):S8.
- Zhou ZR, Liu SX, Zhang TS, Chen LX, Xia J, Hu ZD, Li B. Short-course preoperative radiotherapy with immediate surgery versus long-course chemoradiation with delayed surgery in the treatment of rectal cancer: a systematic review and meta-analysis. Surg Oncol. 2014 Dec;23(4):211-21.
- Latkauskas T, Pauzas H, Gineikiene I, Janciauskiene R, Juozaityte E, Saladzinskas Z, et al. Initial results of a randomized controlled trial comparing clinical and pathological downstaging of rectal cancer after preoperative short-course radiotherapy or long-term chemoradiotherapy, both with delayed surgery. Colorectal Disease: The Official Journal of the Association of Coloproctology of Great Britain and Ireland. 2012;14(3):294–8.
- Bujko K, Kolodziejczyk M. The 5 x 5 Gy with delayed surgery in non-resectable rectal cancer: a new treatment option. Radiotherapy and Oncology: Journal of the European Society for Therapeutic Radiology and Oncology. 2008;87(3):311–3.
- Beppu N, Matsubara N, Noda M, Yamano T, Kakuno A, Doi H, Kamikonya N, Kimura F, Yamanaka N, Yanagi H, Tomita N. Short-course radiotherapy with delayed surgery versus conventional chemoradiotherapy: A comparison of the short- and long-term outcomes in patients with T3 rectal cancer. Surgery. 2015; 158(1):225-35.
- Pettersson D, Holm T, Iversen H, Blomqvist L, Glimelius B, Martling A. Preoperative short-course radiotherapy with delayed surgery in primary rectal cancer. The British Journal of Surgery. 2012; 99(4):577-83.
- Pettersson D, Lörinc E, Holm T, Iversen H, Cedermark B, Glimelius B, Martling A. Tumour regression in the randomized Stockholm III Trial of radiotherapy regimens for rectal cancer. The British Journal of Surgery. 2015; 102(8):972-8; discussion 8.
- Radu C, Berglund A, Pahlman L, Glimelius B. Short-course preoperative radiotherapy with delayed surgery in rectal cancer—a retrospective study. Radiotherapy and Oncology: Journal of the European Society for Therapeutic Radiology and Oncology. 2008;87(3):343–9.
- Hatfield P, Hingorani M, Radhakrishna G, Cooper R, Melcher A, Crellin A, Kwok-Williams M, Sebag-Montefiore D. Short-course radiotherapy, with elective delay prior to surgery, in patients with unresectable rectal cancer who have poor performance status or significant co-morbidity. Radiotherapy and Oncology: Journal of the European Society for Therapeutic Radiology and Oncology. 2009; 92(2):210-4.
- Valentini V, Glimelius B, Haustermans K, Marijnen CA, Rodel C, Gambacorta MA, et al. EURECCA consensus conference highlights about rectal cancer clinical management: the radiation oncologist’s expert review. Radiotherapy and Oncology: Journal of the European Society for Therapeutic Radiology and Oncology. 2014;110(1):195–8.
- Avallone A, Piccirillo MC, Delrio P, Pecori B, Di Gennaro E, Aloj L, Tatangelo F, D'Angelo V, Granata C, Cavalcanti E, Maurea N, Maiolino P, Bianco F, Montano M, Silvestro L, Terranova Barberio M, Roca MS, Di Maio M, Marone P, Botti G, Petrillo A, Daniele G, Lastoria S, Iaffaioli VR, Romano G, Caracò C, Muto P, Gallo C, Perrone F, Budillon A. Phase 1/2 study of valproic acid and short-course radiotherapy plus capecitabine as preoperative treatment in low-moderate risk rectal cancer-V-shoRT-R3 (Valproic acid--short Radiotherapy--rectum 3rd trial). BMC Cancer. 2014 Nov 24;14:875.
- Heo SH, Kim JW, Shin SS, Jeong YY, Kang H-K. Multimodal imaging evaluation in staging of rectal cancer. World Journal of Gastroenterology : WJG. 2014;20(15):4244-4255.
- Fusco R, Sansone M, Petrillo M, Avallone A, Delrio P, Tatangelo F, Petrillo A. Role of Magnetic Resonance Imaging in Locally Advanced Rectal Cancer, Colorectal Cancer - Surgery, Diagnostics and Treatment, Dr. Jim Khan (Ed.), InTech 2014, DOI: 10.5772/56831.
- Beets-Tan RG, Beets GL. Rectal cancer: review with emphasis on MR imaging. Radiology 2004;232(2):335–346.
- Leach MO, Brindle KM, Evelhoch JL, Griffiths JR, Horsman MR, Jackson A, Jayson GC, Judson IR, Knopp MV, Maxwell RJ, McIntyre D, Padhani AR, Price P, Rathbone R, Rustin GJ, Tofts PS, Tozer GM, Vennart W, Waterton JC, Williams SR, Workman P; Pharmacodynamic/Pharmacokinetic Technologies Advisory Committee, Drug Development Office, Cancer Research UK. The assessment of antiangiogenic and antivascular therapies in early-stage clinical trials using magnetic resonance imaging: issues and recommendations. Br J Cancer 2005;92(9):1599–1610.
- Petrillo A, Fusco R, Petrillo M, Granata V, Sansone M, Avallone A, Delrio P, Pecori B, Tatangelo F, Ciliberto G. Standardized Index of Shape (SIS): a quantitative DCE-MRI parameter to discriminate responders by non-responders after neoadjuvant therapy in LARC. Eur Radiol. 2015 Jan 11.
- Petrillo M, Fusco R, Catalano O, Sansone M, Avallone A, Delrio P, Pecori B, Tatangelo F, Petrillo A. MRI for Assessing Response to Neoadjuvant Therapy in Locally Advanced Rectal Cancer Using DCE-MR and DW-MR Data Sets: A Preliminary Report. Biomed Res Int. 2015;2015:514740.
- Beets-Tan RG, Beets GL. MRI for assessing and predicting response to neoadjuvant treatment in rectal cancer. Nat Rev Gastroenterol Hepatol. 2014 Aug;11(8):480-8.
- Phongkitkarun S, Tohmad U, Larbcharoensub N, Sumbunnanondha K, Swangsilpa T, Sirachainan E. DCE-MRI-Derived Parameters as Predictors of Response to Neo-Adjuvant Chemoradiation Treatment of Rectal Carcinoma. J Med Assoc Thai. 2016 Mar;99(3):338-47.
- Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988 Aug;168(2):497-505.
- Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology 1986; 161(2):401-407.
- Oto A, Yang C, Kayhan A, Tretiakova M, Antic T, Schmid-Tannwald C, Eggener S, Karczmar GS, Stadler WM. Diffusion-weighted and dynamic contrast-enhanced MRI of prostate cancer: correlation of quantitative MR parameters with Gleason score and tumor angiogenesis. AJR Am J Roentgenol. 2011 Dec;197(6):1382-90.
- Curvo-Semedo L, Lambregts DM, Maas M, Thywissen T, Mehsen RT, Lammering G, Beets GL, Caseiro-Alves F, Beets-Tan RG. Rectal cancer: assessment of complete response to preoperative combined radiation therapy with chemotherapy--conventional MR volumetry versus diffusion-weighted MR imaging. Radiology. 2011 Sep;260(3):734-43.
- A, Deux J-F, Brugieres P, Rahmouni A. Liver cirrhosis: intravoxel incoherent motion MR imaging-pilot study. Radiology 2008; 249(3): 891-899.
- Wirestam R, Borg M, Brockstedt S, Lindgren A, Holtas S, Stahlberg F. Perfusion-related parameters in intravoxel incoherent motion MR imaging compared with CBV and CBF measured by dynamic susceptibility contrast MR technique. Acta Radiol. 2001; 42(2): 123-128.
- Granata V, Fusco R, Catalano O, Guarino B, Granata F, Tatangelo F, Avallone A, Piccirillo M, Palaia R, Izzo F, Petrillo A. Intravoxel incoherent motion (IVIM) in diffusion-weighted imaging (DWI) for Hepatocellular carcinoma: correlation with histologic grade. Oncotarget. 2016 Nov 29;7(48):79357-79364.
- Granata V, Fusco R, Catalano O, Filice S, Amato DM, Nasti G, Avallone A, Izzo F, Petrillo A. Early Assessment of Colorectal Cancer Patients with Liver Metastases Treated with Antiangiogenic Drugs: The Role of Intravoxel Incoherent Motion in Diffusion-Weighted Imaging. PLoS One. 2015 Nov 13;10(11):e0142876
- Jensen JH, Helpern JA. MRI quantification of non-Gaussian water diffusion by kurtosis analysis. NMR Biomed. 2010; 23:698–710.