2530
Towards efficient free breathing dynamic liver MRI using Cartesian k-space sampling with compressed sensing1Department of Radiology, Zhongshan Hospital, Fudan University, Shanghai, China, 2United Imaging Healthcare, Shanghai, China
Synopsis
An efficient imaging method using Cartesian k-space undersampling with compressed sensing, and automatic detection of respiration was proposed to enable free-breathing dynamic liver imaging with a temporal resolution up to 1.0 sec/phase
Introduction
Dynamic contrast enhanced T1w 3D GRE plays an important role in liver imaging, but may suffer from severe artifacts due to respiratory motion. Golden-angle radial acquisition with compressed sensing reconstruction was reported for dynamic liver MRI with free breathing1,2. However, compared to Cartesian sampling, the time efficiency of k-space coverage in radial trajectories is much lower, especially combined with square in-plane FOV and restricted undersampling along slice dimension in stack-of-stars acquisition. In this work, we propose a method to achieve highly efficient free-breathing dynamic liver imaging with Cartesian k-space undersampling in T1w 3D GRE sequence. The feasibility and performance of the method was evaluated with volunteers.Methods
Data Acquisition: On a T1w 3D GRE sequence, Cartesian k-space was randomly undersampled with Poisson-disc pattern in the plane of two orthogonal phase encoding directions3. Mutual complementary acquisition mode was designed to promote information redundancy therefore improve acceleration capability along temporal axis. For instance, if one view point uncollected in a frame will gain high probability to be collected in the next frame compared to other candidates. Quick fat suppression was achieved by applying a spectrally selective fat-saturation RF pulse followed by a series of imaging echoes.
Reduction of Motion Artifacts: In a transversal liver imaging, the readout axis was switched from R-L to A-P direction compared to conventional acquisition. A one-dimensional navigator was applied along A-P direction before the Fat-Sat RF pulse of each echo train. A training procedure in advance of the image data acquisition was adopted to learn patient’s respiration pattern, and to determine a threshold. During the free breathing, only the image data corresponding to a position within the threshold were acquired with the help of a real-feedback framework, which updates the respiration state and control the data acquisition. Breathhold and external respiration monitor were not required.
Image Reconstruction: An non-linear reconstruction was used to exploit the acceleration ability of both spatial, temporal sparsity and parallel imaging:
argminx(∑||Fu(Cl.x)-yl||2 + λ1TV(x) + λ2TVtemp(x))
Here x denotes the reconstructed multiphase 3D image; Cl denotes the coil sensitivity of channel l; Fu represents the Fourier transform operator with undersampling pattern; TV and TVtemp represents total variation operator in image space and temporal axis respectively; λ is real-valued scaling factor.
Both proposed sequence and reconstruction algorithm were implemented in C++ to support inline data acquisition and image reconstruction. Five volunteers were recruited to perform free breathing dynamic liver imaging with contrast enhancement on a 3T clinical scanner (uMR 770, UIH, Shanghai, China). Acquisition parameters: RO x PE x SPE x Phase = 208 x 150 x 40 x 30, with a net acceleration factor of 11.0, and whole liver coverage; spatial resolution 1.2mm x 2.0 mm x 4.5mm; total acquisition time varying from 2.5 ~ 3.5 minutes dependent on the volunteer’s respiration pattern.
Results
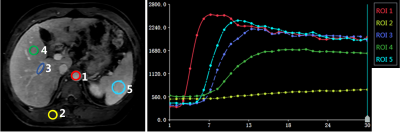
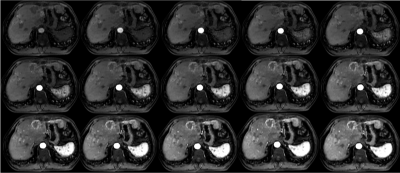
The signal changes as contrast materials transited through tissues were clearly depicted by the 30-phase acquisition (see Fig1). No respiratory artifacts and reconstruction imperfections (streaking artifacts, residual aliasing etc.) were observed. Tissues were almost maintained static over different phases, which was further confirmed by the signal intensity-time curves of good quality (see Fig2).Discussion and Conclusion
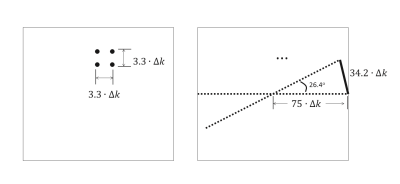
Compared to radial acquisition, Cartesian k-space undersampling is more efficient, therefore leads to better capability for imaging acceleration. As illustrated in Fig3, to obtain a net acceleration factor of 11.0 as in above experiments, only 13.6 in-plane spokes can be acquired in stack-of-stars imaging (given fully sampling along SPE), resulting in an local distance of 34.2∙∆k, between two adjacent points at the periphery part of k-space, whereas only 3.3∙∆k on average in Cartesian undersampling with the same acceleration factor. To suppress the streaking artifacts with more spokes will substantially decrease the temporal resolution.
In above experiments, the temporal resolution was about 2.0sec/phase if without interruption from respiration or in a breathhold acquisition. The temporal resolution can be further improved (e.g. <1.0sec/phase) by slightly reducing the acquisition matrix and increasing the acceleration factor (up to 16.0), as shown in Fig4. With this configuration, at least 1~2 phases can be acquired during each respiratory cycle for most patients in free breathing. In conclusion, the proposed method provides very high acceleration and reliable image quality in 3D dynamic imaging, which does not only enable the capture of rapid signal changes (e.g. in early arterial phase), but also performs as a promising candidate for quantitative analysis in DCE liver perfusion.
Acknowledgements
No acknowledgement found.References
1. Li Feng, Leon Axel, Hersh Chandarana et al. XD-GRASP: Golden-Angle Radial MRI with Reconstruction of Extra Motion-State Dimensions Using Compressed Sensing. Magn Reson Med. 2016; 75(2): 775–788.
2. Hersh Chandarana, Li Feng, Justin Ream et al. Respiratory Motion-Resolved Compressed Sensing Reconstruction of Free-Breathing Radial Acquisition for Dynamic Liver MRI. Invest Radiol. 2015 November ; 50(11): 749–7563.
3. R. Bridson. Fast Poisson disk sampling in arbitrary dimensions. ACM SIGGRAPH 2007 Sketches Program
Figures