2528
Comparison of liver motion measured by dynamic MRI and respiration signals obtained by an optical sensor1Philips Research Laboratories, Hamburg, Germany, 2Philips Innovation Services, Eindhoven, Netherlands, 3Philips Research Laboratories, Eindhoven, Netherlands, 4Philips Healthcare, Gainesville, FL, United States, 5Philips Research North America, Cambridge, MA, United States, 6Department of Radiology and Advanced Imaging Research Center, UT Southwestern Medical Center, Dallas, TX, United States
Synopsis
To monitor the breathing status of a subject and to synchronize data acquisition with respiration, external sensors, such as respiratory bellows, are routinely used. These sensors probe only the local breathing motion, and, hence, their signal quality can vary significantly depending on the individual subject’s physiology and morphology as well as the experience of the MR operator. Recently, optical sensors were proposed as an alternative. The purpose of this work was to compare the signals obtained by an optical sensor and by a pressure-based sensor with respect to their ability to represent the true liver motion during breathing.
Introduction
Coping with respiratory motion is a major challenge in body MRI. To monitor the breathing status of a subject and to synchronize data acquisition with respiration, pressure-based external sensors, such as respiratory bellows, are routinely used. These sensors probe only the local breathing motion, and, hence, the signal quality of such sensors can vary significantly depending on the individual subject’s physiology and morphology as well as the experience of the MR operator. Recently, optical sensors were proposed as an alternative, where video images are processed to extract the displacement of selected body parts induced by breathing1,2. The purpose of this work was to compare the signals obtained by an optical sensor and by a pressure-based sensor with respect to their ability to represent the true liver motion during breathing.Methods
A monochrome camera of type IDS uEye (IDS, Obersulm, Germany) equipped with a 4-12 mm vari-focal lens (Tamron, Saitama, Japan) was installed at the rear wall of the MR examination room at approximately 1.9 m from the isocenter of a 3T Ingenia MRI scanner (Philips Healthcare, Best, Netherlands) and used to acquire video data of the subject torso during MR image acquisition (Fig. 1). The camera was operated at 20 frames per second and was connected to a computer via a USB cable equipped with cable traps to reduce RF-interferences. Breathing signals were derived in real-time from the video data using a fully automated algorithm developed previously for patient monitoring3 and streamed to the MR data acquisition system via the scanner physiology interface. This allowed the acquisition of breathing signals simultaneously with both a pressure-based respiratory bellows (Invivo, Gainesville, USA) and the above described optical sensor, that were stored during scanning in physiology log-files for further analysis.
To measure the liver
motion due to breathing, dynamic single-slice MR images were acquired in 10
volunteers in sagittal orientation using a single-shot SSFP sequence (pixel
size: 1.25x1.25 mm, FOV: 280x280x8 mm, TR / TE = 2.51 / 1.26 ms, flip angle = 20°)
with an anterior torso coil-array and a posterior coil-array embedded in the
patient support. 120 dynamics with a temporal resolution of 223ms were acquired
during regular breathing. The respiratory bellows was positioned on the
subject’s abdomen below the anterior torso coil and was secured using a Velcro
strap. The study was IRB approved and written informed consent
was obtained from all volunteers. During acquisition, timestamps were written
in the physiology log-file to mark the acquisition time of the k-space center
of each individual dynamic.
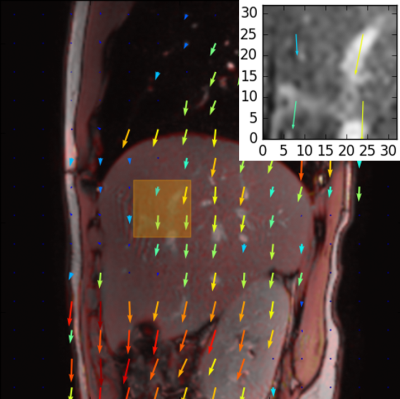
Motion fields were computed for each dynamic of the torso scan using the Gunnar Farnebäck optical flow algorithm4 from openCV 2.4.6. The mean displacement along the feet-head direction was computed within a ROI placed manually in the liver, where the motion field computation was found to be the most robust, and used as a marker of the liver motion during breathing [Fig. 2].
Based on the acquisition markers stored in the physiology log-files, breathing signals from the two external sensors were compared to the motion curve derived from the dynamic MR images. Cross-correlation coefficients between the signals were computed to quantify the ability of each sensor to represent liver motion.
Results
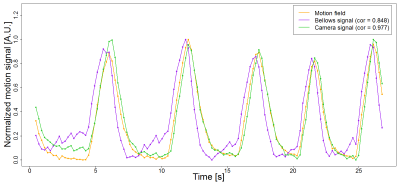
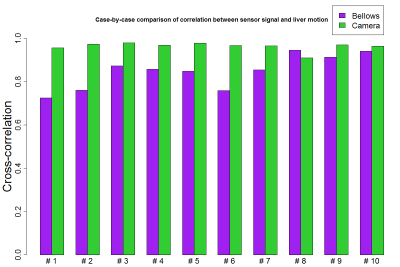
In all experiments, signals measured by the external sensors and the liver motion signals computed from the dynamic scans exhibited the expected inspiration and expiration characteristics of breathing motion and showed comparable respiratory patterns, both in amplitude and frequency (Fig. 3). The correlation between liver motion signal and breathing signals measured by the external sensors was higher on average for the camera sensor, with values in the range [0.91-0.98], than for the respiratory bellows (range [0.72-0.94]). In 9 out of 10 cases, the correlation coefficient of the camera signal was larger than that of the respiratory bellows (Fig. 4).Discussion
Visual inspection of the extracted signals suggested that the higher correlation between camera sensor and liver motion signals was due to a better agreement between peak-to-peak amplitude variations and reduced temporal shift during the expiratory phase. This may indicate that the camera sensor was able to better capture the full torso motion during the expiration, while the respiratory bellows was only sensitive to the local motion of the abdominal wall. Further studies including more subjects with higher variability in morphology and physiology are needed to confirm these preliminary results.Conclusion
Breathing signals obtained by the optical sensor were found to have high correlation with the computed liver motion and to more accurately represent the breathing status than the pressure-based respiratory bellows in a group of 10 healthy volunteers.Acknowledgements
No acknowledgement found.References
- J. Maclaren, M. Aksoy, and R. Bammer. Contact-free Physiological Monitoring Using a Markerless Optical System. Magn Reson Med. 2015; 74(2):571-577.
- J.Y. Cheng, J. Lu, G. Scott et al. Optical Motion Monitoring for Abdominal and Lung Imaging. ISMRM 2017, #3936.
- M. Rocque. Fully Automated Contactless Respiration Monitoring Using a Camera. IEEE International Conference on Consumer Electronics, 2016.
- G. Farnebäck. Two-frame motion estimation based on polynomial expansion. In Image Analysis, pages 363-370, Springer, 2003.
Figures