2527
A study of correlation of the SUVmax and ADC in malignant breast tumors using simultaneous PET-MRI1Medical physics and research department, Hong Kong Sanatorium & Hospital, Happy Valley, Hong Kong, 2Department of diagnostic & interventional radiology, Hong Kong Sanatorium & Hospital, Happy Valley, Hong Kong, 3Department of nuclear medicine &positron emission tomography, Hong Kong Sanatorium & Hospital, Happy Valley, Hong Kong, 4Breast Care Center, Hong Kong Sanatorium & Hospital, Happy Valley, Hong Kong
Synopsis
We studied the correlation of simultaneous DWI-ADC and 18F-FDG SUVmax in invasive ductal carcinoma (IDC) tumors (n=41), and their association with different diagnostic factors using integrated PET-MRI. An insignificant inverse correlation was found (r=-0.214, p=0.179) between SUVmax and ADCmean. SUVmax was significantly associated with tumor T-stage (p=0.024). ADCmean of the index IDC was significantly smaller in the patients with pathologically confirmed regional lymph node metastasis (p=0.0488) and estrogen receptor status (p=0.0254). An insignificantly larger SUVmax (p=0.1352) was found in triple negative IDCs. Our results showed that SUVmax and ADCmean might potentially have complementary roles in breast cancer characterization.
Introduction
DWI-ADC and standardized uptake value (SUV) of 18F-fluorodeoxyglucose-PET (18F-FDG-PET) have been used for characterizing breast cancer (BC) tumors, and are associated with different tumor properties tumor (1). The correlations of ADC and SUV were all studied using individually acquired MRI (in prone) and PET-CT (in supine) data at different time intervals, therefore potentially biased by kinetic variations and positional mismatch (1-4). We aimed to study the correlation of DWI-ADC and 18F-FDG SUVmax in invasive ductal carcinoma (IDC) of breast, and their association with different diagnostic tumor factors using a simultaneous PET-MR scanner.Methods
66 consecutive newly diagnosed female BC patients (47.6±8.6 years) underwent pre-operative PET-MR evaluation (Biograph mMR, Siemens Healthineers) with an attenuation corrected 4-channel breast coil. The regional prone scan began at ~130-150 minutes post 18F-FDG injection with a single-bed 5-minute PET acquisition. MRI protocol included anatomical sequences, pre-contrast DWI (SS-EPI, b=0,1000 s/mm2, TE/TR=77/11200ms, pixel-size=1.4mm, thickness/gap=3/1mm,) and DCE-MRI. ADCmean (excluding cystic and necrotic part) and SUVmax of each index IDC tumor were calculated. DCE-MRI was used as anatomical reference. Pearson correlation coefficient of ADCmean and SUVmax were calculated. The relationship of ADCmean and SUVmax of the index IDC tumor with pathological tumor T-staging and regional lymph node N-staging (AJCC BC staging 7th edition), nuclear grade, and receptor profiles (estrogen receptor ER, progesterone receptor PR, human epidermal growth factor receptor HER2) were assessed.Results
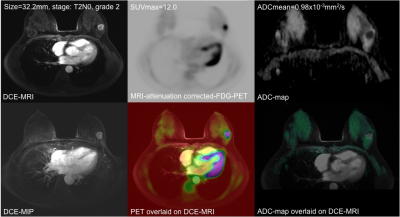
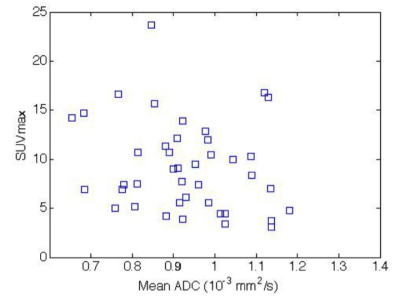
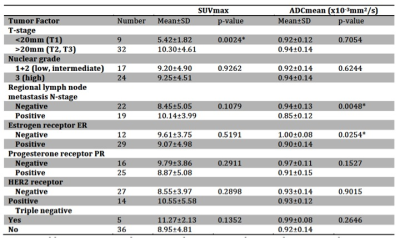
25/66 patients were excluded because of incomplete histopathology, lack of receptor data (n=21), too small lesions (<10mm, n=2) or severe DWI artifacts(n=2). 41/66 patients were histopathologically confirmed to have IDC tumors (46.4±8.8 years). Figure 1 shows simultaneously acquired PET-MR and MR images of a grade-II T2N0 IDC. Figure 2 is the scatter plot of SUVmax and ADCmean of all 41 IDCs, showing insignificant inverse correlation (p=0.179; correlation coefficient r=-0.214) between them. The linear regression line was y=-7.31x +16.04 (R2=0.046). The relationships of SUVmax and ADCmean with various factors were summarized (Table.1). SUVmax was significantly associated with tumor pathological T-stage. The mean SUVmax (10.30±4.61) in pathological T2+T3 tumors were significantly higher (p=0.0024) than that in T1 tumors (5.42±1.82). ADCmean of the index IDC tumor had significant relationship with the pathologically confirmed regional lymph node metastasis (p=0.0488) and ER status (p=0.0254). A larger SUVmax was observed with triple negative IDC but not significantly different from other IDC (11.27±2.13 versus 8.95±4.81; p=0.1352). ADCmean tended to be smaller in PR positive than in PR negative tumors, but not significant (0.91±0.15 versus 0.97±0.11x10-3 mm2/s; p=0.1527).Discussion
Heterogeneous correlation results of ADC and SUV with various factors have been reported for BC by using sequentially acquired MRI and PET/CT (1-4). Acquisition time and patient position difference might lead to bias and mismatch in these correlation studies. To our knowledge, this is the first study to assess BC ADC and SUV simultaneously using a hybrid PET-MR scanner, potentially eliminating the time and position biases. Similar to previous studies, an inverse insignificant correlation was found between SUVmax and ADCmean since both parameters are only partially related to tumor cellularity. SUVmax was significantly associated with tumor T-stage, and reflected enhanced glycolysis in more aggressive tumors. The ADCmean of the index IDC was observed significantly smaller in pathologically confirmed node positive patients than in node negative patients. It is noteworthy that we did not directly measure the ADC and SUV of the metastatic lymph node for two reasons. First, PET-MRI is still subject to low diagnosis accuracy of node metastasis compared to biopsy. Second, some regional lymph nodes were located out of the FOV in the prone PET-MRI scan. ADCmean was significantly associated with ER status, which might be explained by the fact that ER blocks tumor angiogenesis pathway and reduces extra-cellular space and thus increases diffusion restriction (1). In contrast, SUVmax had a greater association than ADCmean with triple negative BC (TNBC), although not statistically significant. By nature of its aggressive biologic behavior and poorer prognosis due to lack of target for hormonal therapy (5), TNBC might be more appropriate to be metabolically characterized by SUV.
The limitations of this study are the inclusion of IDC-only pathology and the known source of errors related to Dixon-segmentation-based MRI attenuation correction. However, the superficial nature of breast tumor pathologies and prone imaging postion have both minimized the errors related to these limitations.
Conclusion
SUVmax and ADCmean were simultaneously assessed in IDC using a hybrid PET-MRI, and an inverse insignificant correlation between them was found. The associations of SUV and ADC with various diagnostic factors indicated that these two parameters could potentially have some partially complementary roles in BC characterization.Acknowledgements
No acknowledgement found.References
1. Baba S, Isoda T, Maruoka Y, et al. Diagnostic and prognostic value of pretreatment SUV in 18F-FDG/PET in breast cancer: comparison with apparent diffusion coefficient from diffusion-weighted MR imaging. J Nucl Med 2014;55(5):736-742.
2. Kitajima K, Yamano T, Fukushima K, et al. Correlation of the SUVmax of FDG-PET and ADC values of diffusion-weighted MR imaging with pathologic prognostic factors in breast carcinoma. Eur J Radiol 2016;85(5):943-949.
3. Karan B, Pourbagher A, Torun N. Diffusion-weighted imaging and (18) F-fluorodeoxyglucose positron emission tomography/computed tomography in breast cancer: Correlation of the apparent diffusion coefficient and maximum standardized uptake values with prognostic factors. J Magn Reson Imaging 2016;43(6):1434-1444.
4. Miyake KK, Nakamoto Y, Kanao S, et al. Journal Club: Diagnostic value of (18)F-FDG PET/CT and MRI in predicting the clinicopathologic subtypes of invasive breast cancer. AJR Am J Roentgenol 2014;203(2):272-279.
5. Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med 2010;363(20):1938-1948.
Figures