2517
eNOS-/- mice fed with HFD develop progressive non-alcoholic fatty liver disease (NAFLD) which is partially reversible with antihypertensive and hypoglycemic therapyBegoña Lavin Plaza1, Marcelo E Andia2, Thomas Eykyn1, Alkystis Phinikaridou1, Aline Xavier2, and Rene M Botnar1
1Imaging Sciences and Biomedical Engineering, King's College London, London, United Kingdom, 2Radiology department, School of Medicine, Pontificia Universidad Catolica de Chile, Santiago, Chile
Synopsis
Liver steatosis or non-alcoholic fatty liver disease (NAFLD) is the most common liver disease in Western countries. However, the cause and treatments are still controversial. Nitric oxide (NO) and its derivatives play important roles in the physiology and pathophysiology of the vascular system and liver metabolism. We quantified intraperitoneal fat and liver fat-fraction using 3T MRI in eNOS-/- mice fed with HFD and investigated (1) whether pharmacological treatments for type 2 diabetes and hypertension reduced fat deposition and (2) if the phenotype could be recapitulated by administration of an inhibitor of endothelial NO synthesis (L-NAME) in wild type mice.
Introduction
Non-alcoholic fatty liver disease (NAFLD) is the most common liver disease in Western countries, and it has been considered the liver expression of the metabolic syndrome1. However, the cause and treatments of NAFLD are still controversial. Nitric oxide (NO) and its derivatives play important roles in the physiology and pathophysiology of the vascular system and liver2. NO availability not only affects the vascular system but also induces changes in mitochondrial bioenergetics that result in increased accumulation of fatty acids in the liver. eNOS-/- animals develop systemic alterations similar to type 2 diabetes and hypertension3. In addition, they also develop progressive NAFLD when fed with high-fat diet (HFD)4. In this work we investigated whether the total amount of intraperitoneal fat and liver fat-fraction in eNOS-/- mice fed with HFD is ameliorated by pharmacological treatments for type 2 diabetes (Metformin) and hypertension (Losartan). Additionally, we studied if the administration of an inhibitor of endothelial NO synthesis (L-NAME)5 in wild type mice induces the same phenotype as observed in eNOS-/- mice thereby establishing whether nitric oxide plays an important role in the development of NAFLD.Methods
Study design is summarized in Fig.1. In vivo MRI: Whole body 3-point Dixon images were obtained using a 3T clinical MR scanner (Achieva, Philips Healthcare, Best, NL) and a 47mm single-loop surface coil. The acquisition parameters include TR/ TE1/ TE2/ TE3 = 15/ 2.3/ 3.5/ 4.7 ms; flip angle 10º; in plane resolution= 0.17 x 0.17 mm; slice thickness= 0.5 mm. Animal treatments: All treatments were administered in water as follow: L-NAME (0.5 mg/mL), Metformin (2 mg/mL), Losartan (0.3 mg/mL).Results
eNOS-/- mice fed with HFD have significantly more intraperitoneal fat and liver fat-fraction than WT and eNOS-/- mice fed with normal chow (Fig. 2, Fig. 3A, Fig. 4A). WT mice that received L-NAME have similar phenotype as in eNOS-/- mice (Fig. 3A and Fig. 3B). Lower amounts of intraperitoneal fat and liver fat-fraction were observed in eNOS-/- mice fed with HFD and treated with Losartan and Metformin, with a slightly synergic effect when both treatments were administered together (Fig. 3B and Fig. 4B). Our results suggest that the treatment of hypertension and type 2 diabetes induced by the lack of eNOS, normalize the fat liver content.Conclusion
Using in vivo MRI we demonstrated that liver fatty acid accumulation is accelerated in eNOS-/- mice fed with HFD, which could be replicated in WT mice fed with L-NAME. Intraperitoneal and liver fat accumulation could be partially normalized after the administration of losartan and metformin. Our results show the relevance of noninvasive monitoring of liver fat fraction and body composition to evaluate the response and adherence to pharmacological treatments.Acknowledgements
The British Heart Foundation (RG/12/1/29262). CONICYT-PIA, Anillo ACT1416. PuenteUC P1712/2017.References
1Cohen JC, Science 2011; 2Iwakiri Y, Trends Pharmacol Sci 2015; 3Takahashi T, J Diabetes Res 2014; 4Sheldon RD, J Appl Physiol 2014; 5Suda O, Circulation 2002Figures
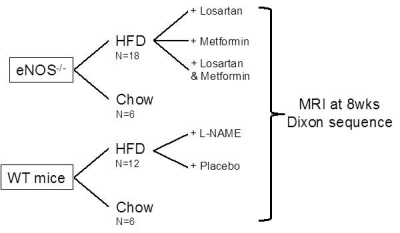
Figure 1- Study design
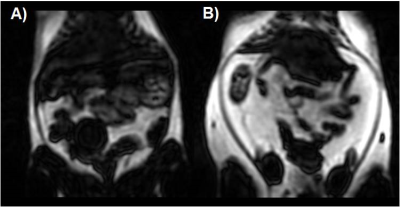
Figure 2- Fat-only images from Dixon acquisition of eNOS-/-
mice fed with (A) normal chow, and (B) HFD for 8wks.
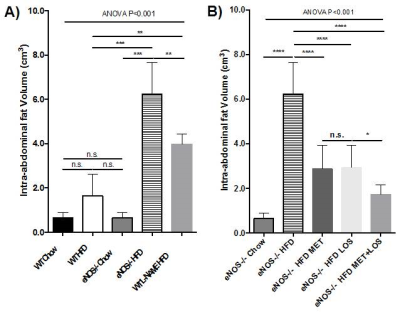
Figure 3- Intra-abdominal fat volume obtained from
3-point Dixon sequence in WT and eNOS-/- mice fed with normal chow
and HFD; and treated with Losartan and/or Metformin.
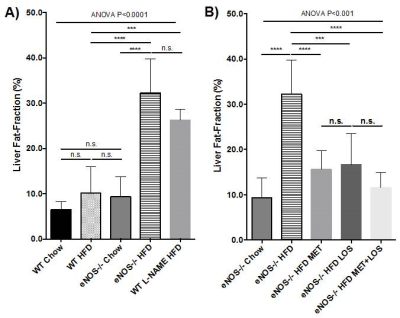
Figure 4- Liver fat-fraction obtained from 3-point
Dixon sequence in WT and eNOS-/- mice fed with normal chow and HFD;
and treated with Losartan and/or Metformin.