2515
Whole-Body Cardio-Respiratory Synchronised DCE-MRI in the Mouse1University of Oxford, CRUK/MRC Oxford Institute for Radiation Oncology, Oxford, United Kingdom
Synopsis
Prospective gating of constant, short TR scans enables rapid imaging to be performed in conjunction with cardiac and respiratory synchronisation. We show that prospectively-gated, dynamic contrast enhanced MRI (DCE-MRI) can be performed over the whole mouse body with a time resolution of ca. 15 s/frame such that multiple organs can be examined simultaneously.
Introduction
Whole body imaging is possible in the mouse as the entire mouse can be located within the homogeneous volume of most magnets, within the acceptably linear volume of most gradient sets, and within the most-homogeneous regions of many RF coils. Whole body imaging necessarily requires cardiac and respiratory synchronisation to avoid motion artefacts derived from these cyclic motions. Dynamic, contrast-enhanced MRI (DCE-MRI) examines the time-dependent delivery and removal of contrast agents that are delivered partway through a repeated scan, which is typically performed using a short TR, T1-weighted imaging. Cardio-respiratory synchronisation in short TR scans has usually been achieved using retrospective gating1 but as this technique requires multiple samples of the same k-space data, it is necessarily slow. We have previously shown that accelerations are possible using prospective cardio-respiratory control in conjunction with k-space segmentation2,3,4 and we now show that cardio-respiratory motion desensitised DCE-MRI can be performed with coverage over the whole mouse.Methods
MRI was performed at 4.7 T with useable magnet, gradient and RF coil volumes of >20 cm (sphere), 8 cm (sphere) and 25*100 mm (diameter*length) RF coil volumes respectively. ECG was detected using subcutaneously-implanted needles placed under the skin of the thorax, and respiration was detected using a pressure balloon. Gating signals were conditioned such that only heartbeats measured during the interbreath rest periods were used for imaging and data acquired from the 2 heartbeats acquired prior to each breath were reacquired at the end of the same breath. At all times when imaging data were not being acquired, RF and gradient pulsing were applied to maintain the steady state. Mice (n=6) were anaesthetised (1-3% isoflurane in air/O2) and placed in a cradle with temperature and respiration maintained at 35-36°C and 40-60 breaths/minute respectively. DCE was performed using 3D gradient and RF-spoiled FLASH (TE=0.6, TR=1.5, FA=5) at an isotropic 420μm resolution. 64 centre-out fast phase-encode lines were acquired per successful r-wave giving a scan time of ca. 10-15 s/frame. Contrast agent (100 μl, Gadospin-P, Viscover) was infused over 15 s at the start of frame 11/50.Results
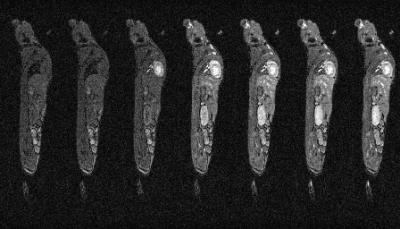
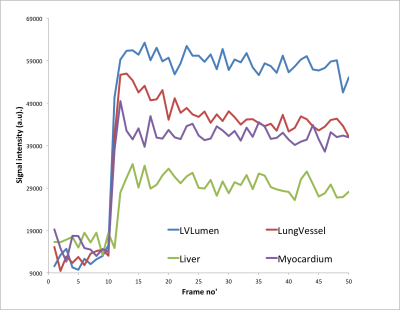
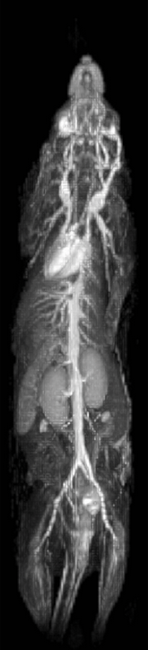
Very stable whole body images can be produced quickly using prospective gating control and contrast agent uptake curves can be formed from static and highly mobile organs, such as heart, liver and lung. Fig. 1 shows a slice from 7 consecutively acquired frames starting from 2 frames before Gd-delivery. Fig. 2 shows Gd-uptake curves from heart, lung and liver. Fig 3 shows the whole body vasculature using a MIP of the sum of all of the dynamically-acquired images.Discussion
The use of prospective cardio-respiratory gating control allows DCE to be performed in multiple organs simultaneously. The centre-out collection of each fast phase-encoding loop within heartbeats enables fast scanning to be performed thereby making the method applicable to the examination of the rapid dynamic changes that occur in DCE-MRI. The automatic reacquisition of data corrupted upon entry to any breath at the end of the same breath ensures effects of respiration are minimised and that k-space amplitude discontinuities arising from rapidly changing T1 are minimised. As a consequence intensities are mapped with good accuracy and precision, and the method is suitable for the quantitative examination of multifocal disease and processes. Different uptake kinetics were observed in different organs and repeats of this work using different contrast agents gave different results, though within groups receiving the same contrast agents as each other results were similar. The method is immediately applicable to the generation of angiographic image presentations which may have applications in a number of vascular disease areas.CONCLUSION
Prospective-gating enables fast imaging that can be desensitised to the cardiac and respiratory motions and is suitable for use in the examination of rapidly changing, dynamic processes, such as contrast agent uptake over the whole mouse body.Acknowledgements
This work was supported financially by Cancer Research UK (C5255/A12678, C2522/A10339), and Medical Research Council Unit Grant for Oxford Institute for Radiation Oncology.References
1. Bishop J, Feintuch A, Bock NA, Nieman B, Dazai J, Davidson L, et al. Retrospective gating for mouse cardiac MRI. Magn Reson Med. 2006;55(3):472-7.
2. Kersemans V, Allen PD, Beech JS, Gilchrist S, Kinchesh P, Smart SC. Application of Prospective Cardio-Respiratory Gating for Simultaneous Quantitative DCE-MRI of Multiple Mammary Tumours in the Mouse.Proc Intl Soc Mag Reson Med 2014; 4034.
3. Kinchesh P, Gilchrist S, Gomes A, Kersemans V, Beech J, Allen D, Smart S. Accelerated Imaging of the Mouse Body Using K-Space Segmentation, Cardio-Respiratory Synchronisation and Short, Constant TR: Application to B-SSFP. Proc Intl Soc Mag Reson Med 2016;24:1825.
4. Kinchesh P, Allen P, Beech J, Gilchrist S, Gomes A, Kersemans V et al. Whole Body T1 Mapping of Small Animals Using Prospective Gating and Variable Flip Angle Imaging.Proc Intl Soc Mag Reson Med 2017:3716.
Figures