2514
Mn2+-free chow reduces gastrointestinal signal for T1-weighted MRI of the mouse abdomen1University of Oxford, CRUK/MRC Oxford Institute for Radiation Oncology, Oxford, United Kingdom
Synopsis
Standard commercial chow given to laboratory animals may contain high levels of paramagnetic Mn2+-ions which act as a T1-reducing contrast agent. Signal intensities where Mn2+ is present are increased when using short-TR, T1W-MRI imaging and the GI-tract appears brighter than the rest of the body. As peristalsis is an inherently unstable motional process, high intensity and temporally unstable signals are formed in the GI-tract, creating image-ghosting and decreasing resolution from that prescribed. We present images acquired before and after transition from Mn2+-bearing to Mn2+-free food to show that these deleterious image effects can be reduced through simple dietary formulation change.
INTRODUCTION
Commercial chow is designed to provide a complete, nutrient-rich diet, but may contain 100 mg/kg Mn2+, a mineral which acts as a relaxation time shortening contrast agent for both T1 and T2. As a consequence, gut regions containing this food appear bright in short TE and TR, T1WI. To avoid the problem, various methods of GI-tract modulation including the use of intestinal cleansing with laxatives and dietary changes have been reported, and the use of mashed potato in a transient dietary change made prior to imaging has been proposed as a satisfactory means for achieving this1. The dietary need for Mn2+ is low and adequate delivery can be made through its presence in the drinking water2. In this study we report on the use of a commercially available Mn2+-free chow that reduces intensities on short TR, T1WI which can be given to laboratory animals long term as it provides an otherwise complete diet.METHODS
Naïve CBA mice (n=5) were used, and tap water and food were freely available. Mice were fed standard Mn2+-bearing chow (Teklad Global 18% protein rodent diet, 2918; Envigo) or Mn2+-free chow (Teklad TD140857; Envigo).
MRI was performed at 7 T (Varian VNMRS) using a 26 mm diameter, 100 mm long RF coil (Rapid Biomedical). 20 repeats of whole-body, cardio-respiratory gated, T1-weighted, 3D spoiled gradient echo imaging (TE=1 ms, TR=2.8 ms, FA=5°) at an isotropic resolution of ca. 420 μm were performed over ca. 10 minutes. Anaesthesia was induced and maintained with 1-4% isoflurane in oxygen-enriched air. Rectal temperature was monitored and maintained at 35°C. Subcutaneously-implanted needles and a pressure balloon were used to record and generate cardio-respiratory gating control signals. Imaging was performed at approximately 0, 24, 72 and 96 hours from the start of the experiment with food switched from normal Mn2+-bearing to Mn2+-free chow after the second imaging session at 24 hours.
Single frame anatomical images, and images representing the standard deviation of mean for the time resolved scans were produced (ImageJ) for each animal and imaging session.
RESULTS
Images produced after feeding with Mn2+-bearing chow showed multiple regions of hyperintensity that were reduced in number and intensity after switching the same animals to the Mn2+-free chow. Fig. 1 shows single slices, at approximately the same anatomical level, taken from the 5 mice on 4 occasions; 2 after the transition from Mn2+-bearing to Mn2+-free food. Peristalsis-derived instabilities, for the same slices as per Fig. 1, are presented in Fig. 2 and show a marked reduction when Mn2+-free food is given. Some low-level hyperintensities were still seen in the GI-tract 48 hours after the change of chow and these were reduced a further 24 hours later.DISCUSSION
A nutrient-rich, standard commercial animal foodstuff has been shown to reduce signal derived from the GI-tract in short TR, T1-weighted imaging. Maintenance of minimum delivery of Mn2+ can be achieved using levels of Mn2+ typically found in tapwater so this diet can be maintained indefinitely. This diet avoids the need for invasive procedures for gut voiding and can be provided ad libitum so animals can be maintained with no need for diet change before imaging. As no preparation is required animals may be selected for imaging without notice, and do not need to be removed from their home cages and cage-mates whilst standard chow is eliminated. Hyperintensities were observed at 48 hours after changing foodstuff suggesting that GI-transit may be somewhat slower than previously reported3. Finally, the stability of the intensity in the GI-tract was increased after the change of diet but this was presumably a result of reduced food-related intensity rather than altered gut function.CONCLUSION
Replacement of standard chow with commercially-available Mn2+-free chow can improve resolution and stability in T1-weighted imaging of the GI-tract whilst maintaining dietary input.Acknowledgements
This work was supported by Cancer Research UK (C5255/A12678 and C2522/A10339) and the Medical Research Council .References
1. Kiryu S, Inoue Y, Yoshikawa K, et al. (2010) Diet and gastrointestinal signal on T1-weighted magnetic resonance imaging of mice. Magn Reson Imaging, 28(2):273-80. doi: 10.1016/j.mri.2009.10.005.
2. Institute of Medicine (US) Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington (DC): National Academies Press (US); 2001. 10, Manganese.
3. Schwarz, R., Kaspar, A., Seelig, J et al (2002), Gastrointestinal transit times in mice and humans measured with 27Al and 19F nuclear magnetic resonance. Magn. Reson. Med., 48: 255–261. doi:10.1002/mrm.10207.
Figures
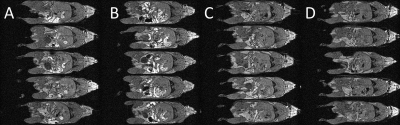
Figure 1: Whole body cardio-respiratory gated T1WI MRI of the mouse. A single slice through the abdomen for each mouse (n = 5, one mouse per row) is shown.
Each column represents a different imaging session; (A) 0 h, Mn2+-bearing chow, (B) 24 h, Mn2+-bearing chow, (C) 72 h, Mn2+-free chow and (D) (C) 96 h, Mn2+-free chow.
The food was switched from normal Mn2+-bearing to Mn2+-free chow after the second imaging session at 24 hours.
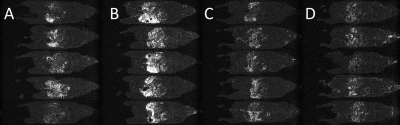
Figure 2: Images representing the standard deviation of the mean for 20 scan repetitions in a single slice through the abdomen for 5 different mice (one mouse per row). The slices shown corresponds to those in Figure 1.
Each column represent a different imaging session; (A) 0 h, Mn2+-bearing chow, (B) 24 h, Mn2+-bearing chow, (C) 72 h, Mn2+-free chow and (D) (C) 96 h, Mn2+-free chow.
The food was switched from normal Mn2+-bearing to Mn2+-free chow after the second imaging session at 24 hours.