2503
Metabolic Imaging of brown adipose tissue in leucine deficient diet fed mice.1Laboratory of Molecular Imaging, Singapore Bioimaging Consortium, Singapore, Singapore
Synopsis
Brown adipose tissue plays an important role in energy expenditure. The deficiency of the essential amino acid leucine has been linked with CREB/TRH pathway and regulation of energy expenditure and food intake. Here we investigated the effect of leucine deficient diet on interscapular brown adipose tissue (iBAT) in mice. Dixon imaging was performed to assess fat fraction changes within iBAT followed by RNA analysis. There was a decrease in fat fraction for leucine deficient diet fed mice together with increased UCP1 expression indicating that short term leucine deprivation leads to iBAT activation.
Purpose
Obesity is caused by impaired balance between food intake and energy expenditure. There is a large interest in mechanisms that regulate energy expenditure through brown adipose tissues [1]. Various molecules have been identified to activate and/or expand brown adipose tissues [2]. The deficiency of the essential amino acid leucine has been linked with CREB/TRH pathway and regulation of energy expenditure and food intake [3]. In this work we investigated the effect of leucine deficient diet on interscapular brown adipose tissue in mice.Methods
Animal experiments were approved by the institutional animal care and use committee. The C57BL/6 (male, 12-16 weeks old) mice were randomized into two cohorts fed with different diets including control n=5 or leucine-deficient (leu (-)) n=5 for 14 days. In vivo imaging was performed using a 7T ClinScan MRI/MRS scanner (Bruker) with a mouse body coil. Dixon imaging was performed to acquire water and fat images from the interscapular region with TR= 8, 24 slices, TE (opposite phase) = 1, TE (in phase) = 2.5, flip angle = 8°. Rolling ball background subtraction algorithm and the percentile based automatic thresholding method were performed to create the mask [4]. Extra unwanted regions were removed and holes inside of the mask were filled by using morphological operation (erosion and dilation). Fat fraction from the water and fat images using fat/ (fat + water) was calculated by using the background removed images. The body fat tissues were collected and weights were recorded. Total RNA was isolated from the iBAT tissues using RNeasy Lipid Tissue Mini Kit (Qiagen 74804).Results
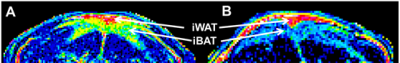
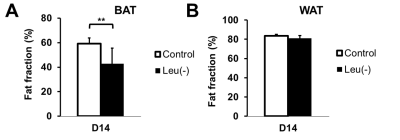
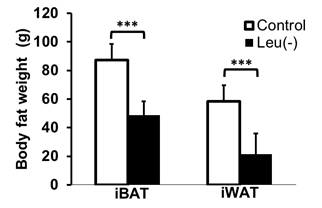
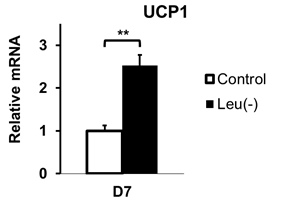
Figure 1 shows representative slices of fat fraction images from interscapular area in control (A) and leu (-) (B) groups respectively. There was a significant reduction in fat fraction within iBAT after 14 days of leucine-deficient diet intervention compared to the control group (Fig2A). Moreover, no differences in fat fraction were identified in iWAT for both study groups (Fig2B). Dissection of different fat compartments revealed significant decrease in both iBAT and iWAT mass under leucine deficiency (Fig.3). Quantitative mRNA analysis showed significantly higher levels of UCP1 expression in iBAT of leu (-) group compared to the control group (Fig4).Discussion
Brown adipose tissue plays an important role in energy expenditure. Decrease in fat fraction within iBAT under leucine deficient diet indicates changes in the properties of the tissue, suggesting mobilization of the lipids. Together with the increased UCP1 expression in the leu (-) group, which is known to be the marker of the brown fat activation, implying activation of brown adipose tissue under leucine deficient condition. The results align with previously reported upregulation of UCP1 in brown adipose tissue due to leucine deprivation [5]. Additionally, there is a reduction in both iBAT and iWAT fat mass in leu (-) group. Decrease in iWAT volume with no change in fat fraction values in leu (-) mice indicates overall mobilization of lipids with no changes to tissue microenvironment .Conclusion
Our results indicate that short term leucine deprivation leads to interscapular brown fat activation with following changes in energy expenditure regulation mechanisms.Acknowledgements
No acknowledgement found.References
1. Saito, M., Brown adipose tissue as a regulator of energy expenditure and body fat in humans. Diabetes Metab J, 2013. 37(1): p. 22-9.
2. Betz, M.J. and S. Enerback, Human Brown Adipose Tissue: What We Have Learned So Far. Diabetes, 2015. 64(7): p. 2352-60.
3. Xia, T., et al., CREB/TRH pathway in the central nervous system regulates energy expenditure in response to deprivation of an essential amino acid. Int J Obes (Lond), 2015. 39(1): p. 105-13.
4. Schneider, C.A., W.S. Rasband, and K.W. Eliceiri, NIH Image to ImageJ: 25 years of image analysis. Nat Methods, 2012. 9(7): p. 671-5.
5. Cheng, Y., et al., Leucine deprivation decreases fat mass by stimulation of lipolysis in white adipose tissue and upregulation of uncoupling protein 1 (UCP1) in brown adipose tissue. Diabetes, 2010. 59(1): p. 17-25.
Figures