2492
Compensating for Bulk Motion in Feed and Wrap Renal Dynamic Radial VIBE DCE-MRI using Bulk Motion Removal and Non-Rigid Registration1Boston Children's Hospital and Harvard Medical School, Boston, MA, United States
Synopsis
Dynamic Radial VIBE DCE-MRI enables motion-robust imaging with high spatiotemporal resolution for accurate estimation of kidney function. However, in feed and wrap DCE-MRI, bulk motion during infant’s sleep reduces the quality of images affected by motion and limits clinical utility of this method for imaging without sedation. This work evaluated the ability of detecting bulk motion using the center-of-k-space line, removing corrupted volumes, and compensating for motion using non-rigid registration for improved parameter estimation accuracy. Results showed that volumes affected by motion were successfully detected and removed in all patients, and the goodness-of-fit to the tracer kinetic model was improved.
Purpose
To evaluate if the effect of bulk motion in feed and wrap (FW) Dynamic Radial VIBE (DRV) DCE-MRI can be compensated for by using bulk motion removal and non-rigid registration, and if this technique improves the glomerular filtration rate (GFR) parameter estimation.Introduction
Dynamic contrast-enhanced (DCE) MRI can be used to measure single kidney glomerular filtration rate (GFR) and differential renal function (DRF) in babies with antenatally detected hydronephrosis. DRF and GFR are used by clinicians to decide who is likely to benefit from surgery. Sedation is required to eliminate infant motion in the scanner. The risk associated with sedation is a limiting factor. Therefore we aim to perform FW DCE-MRI without sedation by compensating for subject motion. The effect of respiratory motion can be minimized using a Dynamic Radial VIBE (DRV) sequence1. However, bulk motion of infant subjects during sleep prevents accurate GFR estimation. In this work we propose to use the center of k-space line to detect and remove DRV volumes corrupted by bulk motion, compensate for the effect of motion using non-rigid registration, and compare our motion-corrected filtration rate parameter estimation to standard DRV.Methods
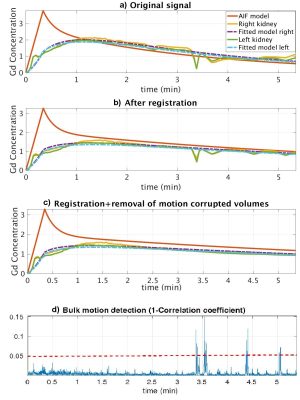
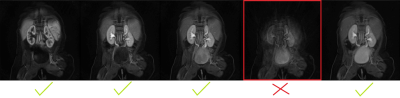
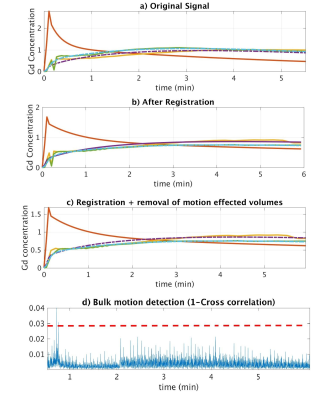
FW DCE-MRIs of 4 infants (1.9-4.5months) and non-FW imaging of 2 children were performed at 3T using Gadavist. Infants were fed, swaddled and rocked to sleep. Images were then acquired for six minutes using a radial “stack-of-stars” 3D FLASH sequence (TR/TE/FA 3.56/1.39ms/12o, 32 coronal slices, voxel size=1.25x1.25x3mm). The mean temporal resolution was 3.3 sec for the arterial phase (2 minutes) and 13 sec for the remaining phases (4 minutes). Bulk motion events were present in the image series. We first registered each volume in the dynamic image series to a reference volume in image space2. The center of k-space point of each radial line was then used to detect motion3,4. The center of k-space points in 3D corresponds to the center of a k-space line. We first applied principal component (PC) analysis to the center of k-space line and kept 2 PCs from each channel. We then constructed the measurement vector (m(t)) of size 2xnumber of channels at each time point t. We computed the correlation coefficient (CC(t)) between m(t) and m(t-1). 1-CC(t) was used to detect motion5. 1-CC(t) larger than a selected threshold indicated a motion event. Volumes corrupted by bulk motion were removed before tracer kinetic (TC) model fitting6 and filtration rate parameter estimation.Results
We compared the model fitting performance of the original signal without motion compensation (MC) with signal after motion compensation with non-rigid registration and after additional compensation with removal of bulk motion in Figure 1. The model-fitting root mean square (RMS) errors were reduced from 0.21 and 0.16 to 0.12 and 0.12 for right and left kidneys respectively after non-rigid registration. The volumes corrupted by bulk motion were removed (Figure 2). The errors were further reduced to 0.09 and 0.09 after removal of bulk motion. In all 6 cases, motion-corrupted volumes were successfully detected and removed resulting in improved model fitting and parameter estimation.Conclusions
We demonstrated that the bulk motion events during feed and wrap DCE-MRI reduce the accuracy of TC model estimation and the reliability of computation of important clinical markers of kidney function (GFR and DRF). The model-fitting error was reduced after removal of volumes affected by bulk motion and registration of volumes to a common reference image. The proposed DRV bulk motion detection and removal method is a potentially useful method for non-sedated imaging of infants with hydronephrosis and potentially impaired renal function without exposing patient to radiation, as opposed to clinical nuclear scintigraphy.Acknowledgements
This work is supported by Crohn’s and Colitis Foundation of America’s Career Development Award, the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the NIH under award R01DK100404 and by the National Institute of Biomedical Imaging and Bioengineering (NIBIB) of the NIH under award R01EB019483.References
1. Feng L, Grimm R, Tobias Block K, et al. Golden-angle radial sparse parallel MRI: Combination of compressed sensing, parallel imaging, and golden-angle radial sampling for fast and flexible dynamic volumetric MRI. Magn Reson Med. 2014;72: 707–717.
2. Commowick, O., Wiest-Daessl´e, N., Prima, S., 2012. Automated di↵eomor- 529 phic registration of anatomical structures with rigid parts: Application to 530 dynamic cervical MRI, in: MICCAI. Springer, pp. 163–170.
3. Cruz, G., Atkinson, D., Buerger, C., Schaeffter, T. and Prieto, C., 2016. Accelerated motion corrected three‐dimensional abdominal MRI using total variation regularized SENSE reconstruction. Magnetic resonance in medicine, 75(4), pp.1484-1498.Vancouver
4. Feng, L., Axel, L., Chandarana, H., Block, K.T., Sodickson, D.K. and Otazo, R., 2016. XD‐GRASP: Golden‐angle radial MRI with reconstruction of extra motion‐state dimensions using compressed sensing. Magnetic resonance in medicine, 75(2), pp.775-788.
5. B. Stemkens et al., “Adaptive bulk motion exclusion for improved robustness of abdominal magnetic resonance imaging,” NMR Biomed., vol. 30, no. August, p. e3830, 2017.
6. Sourbron, Steven P., et al. "MRI-measurement of perfusion and glomerular filtration in the human kidney with a separable compartment model." Investigative radiology 43.1 (2008): 40-48.
Figures