2487
Quantitation of metabolites in human tumour (paraganglioma and GIST) tissues with mitochondrial mutations (SDH and IDH1) by HRMAS 1H NMR spectroscopy1Imaging Core, Cancer Research UK Cambridge Institute, Cambridge, United Kingdom, 2Department of Medical Genetics, University of Cambridge, Cambridge, United Kingdom, 3Department of Endocrinology, Cambridge University Hospital NHS Foundation Trust, Cambridge, United Kingdom, 4Department of Histopathology, Cambridge University Hospital NHS Foundation Trust, Cambridge, United Kingdom, 5Department of Medical Oncology, Cambridge University Hospital NHS Foundation Trust, Cambridge, United Kingdom, 6Department of Radiology, Cambridge University NHS Foundation Trust, Cambridge, United Kingdom
Synopsis
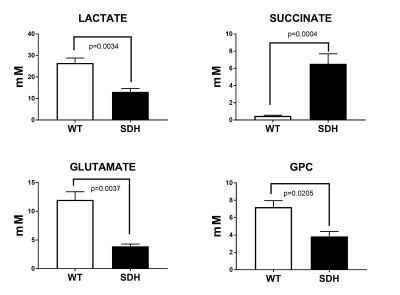
In this study we report, for the first time, the detection of 2HG in IDH1 mutated human GIST tumour tissues by HRMAS 1H NMR spectroscopy. We quantified the levels of Succinate and 2HG in human paraganglioma and GIST tissues. The lactate, glutamate and glycero-phosphocholine (GPC) concentrations were significantly lower in SDHx mutated tumours compared to wild type (WT) tumour tissues, Detection of higher levels of Succinate in SDH mutated tumour tissue and 2HG in IDH1 mutated tissue and their quantitation will be helpful in the stratification of patient treatment in the clinics.
Introduction
The Warburg original hypothesis, that mitochondrial defects are at the root cause of tumorigenesis, regained prominence from the recent findings of mutations in enzymes of TCA cycle. Recent discoveries in mutations in TCA cycle enzymes succinate dehydrogenase (SDH)1, 2, fumarate hydratase (FH)3 and isocitrate dehydrogenase (IDH)4, reinforced the idea that the link between mitochondrial defects and cancer. The tumours with SDH accumulate succinate that can be detected in vivo and ex vivo by NMR due to their concentration levels being in the mill molar range. Mutations in IDH1 gene lead to a neomorphic ability to produce an ‘onco-metabolite’ called 2-hydroxyglutarate (2HG). In this study, we present the quantitation of these metabolites in human cancer (paraganglioma and GIST) tissues with germline mutations (SDHx) and a single tumour with a somatic IDH1 mutation by HRMAS 1H NMR spectroscopy.Methods
Human surgical samples from cancer patients with paraganglioma (WT =18, SDH=6 and IDH1 =1) were preserved at -80°C until NMR analysis. HRMAS 1H NMR data acquisition was performed on a 600 MHz instrument with a 4 mm HRMAS probe (Bruker, Germany). All the spectra were obtained using the TOPSPIN 2.3 software (Bruker, Germany) and at a spin rate of 3000 Hz and a sample temperature of 4°C. A water-suppressed pulse sequence with a repetition time of 8 sec, 128 transients and 64K time domain points was used to acquire the metabolite spectrum. The corresponding water spectrum was acquired with 8 s repetition time, 8 transients and 64K time domain points. A water-suppressed CPMG pulse sequence was used with a T2 filter (50/100/200 ms) to acquire metabolite spectra with suppression of the broad lipid and macromolecule signals; acquisition parameters: 8 s repetition time, 128 transients, 64K time domain points, total analysis time per sample was approximately 90 min. Absolute metabolite concentrations were estimated by fitting the metabolite signals in the water-suppressed HRMAS 1H NMR spectrum in LCModel and using tissue water signal as an internal standard for absolute concentration5.Results
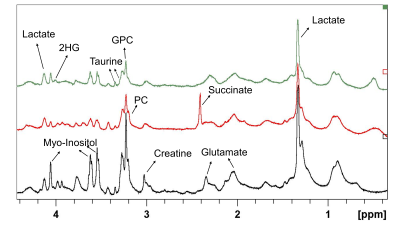
Figure 1 shows representative HRMAS 1H NMR spectra from wildtype, SDH mutated and IDH1 mutated human tumour tissue samples. The lactate, glutamate and glycero-phosphocholine (GPC) concentrations were significantly lower in SDH mutated tumours compared to wild type (WT) tumour tissues, whereas succinate was several folds higher in SDHx mutated tumours tissues (figure 2).Discussion
Significantly lower lactate indicates downregulation of glycolysis in SDHx mutated tumours compared to WT tumours. Lower glutamate, aspartate and choline containing compounds (GPC and t-Choline) indicate reduced amino-acid and membrane phospholipid metabolism in SDHx mutated tumours. A single paraganglioma sample was found to have 2HG accumulation and was identified as harbouring a somatic IDH1 mutation. Two further GIST tumour samples also demonstrated a 2HG peak and further genetic sequencing is being undertaken for a suspected IDH1 mutation. The huge accumulation of succinate detected in the paraganglioma/ GIST tissues was due to the result of SDHx mutation and also similarly the formation of 2HG was due to neomorphic enzyme ability of IDH1 mutation.Conclusions
Detection of higher levels of succinate in SDHx mutated tumour tissue and 2HG in IDH1 mutated tissue and their quantitation will be helpful in the stratification of patient treatment in the clinics. This is the first time that 2HG was found in GIST tissues and further sequencing analysis is underway to explain this accumulation.Acknowledgements
We would like to thank the following funding bodies; Health Research Board Ireland, GIST support UK and Cancer research UK. The authors would like to thank Dr. Stephen Provencher for helping with the simulated basis set used in spectral fitting.References
1.Baysal B.E., Ferrell R.E., Willett-Brozick J. et al. Mutations in sdhd, a mitochondrial complex ii gene, in hereditary paraganglioma. Science 2000, 287, 848-851.
2.Astuti D., Latif F., Dallol A. et al. Gene mutations in the succinate dehydrogenase subunit sdhb cause susceptibility to familial pheochromocytoma and to familial paraganglioma. Am J Hum Genet 2001, 69, 49-54.
3. Tomlinson I.P., Alam N.A., Rowan A.J. et al. Germline mutations in fh predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat Genet 2002, 30, 406-410.
4.Parsons D.W., Jones S., Zhang X. et al. An integrated genomic analysis of human glioblastoma multiforme. Science 2008, 321, 1807-1812.
5.Madhu B, Shaw GL, Warren AY, et al. Response of Degarelix treatment in human prostate cancer monitored by HR-MAS 1H NMR spectroscopy. Metabolomics. 2016; 12:120.