2479
Absolute Reference for Dissolved-Phase 129Xe Spectroscopy Leads to Peak Reassignment1Physics and Astronomy, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States, 2Biomedical Research Imaging Center, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States, 3Applied Physical Sciences, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States
Synopsis
Dissolved-phase 129Xe (DPXe) chemical shift (CS) measurements could benefit from a robust reference system that can provide consistent CS values independently of gas partial pressures, lung inflation, subject position, and shimming conditions. We demonstrate that, by referencing the DPXe frequency to that of nearby protons, consistent CS values can be obtained, both in vitro and in vivo, enabling correct assignment of some of the spectral lines observed in vivo.
Introduction
129Xe is an ideal probe for biological MR applications thanks to its relatively high solubility in tissues and wide chemical shift (CS) range.1 Recently, dissolved-phase 129Xe (DPXe) has been used as a background-free source of contrast for MR studies of brain perfusion,2 tissue oxygenation,3 brown adipose tissue,4 kidney function,5 and temperature imaging.6,7 These studies rely heavily on the ability to identify DPXe peaks and measure their shift with respect to a stable reference. Yet, DPXe CS values, measured with respect to the gas-phase 129Xe resonance, are typically reported with a variation of more than 2-3 ppm. In this work, we assess the use of protons as an internal reference for DPXe CS values in vivo and in vitro.Methods
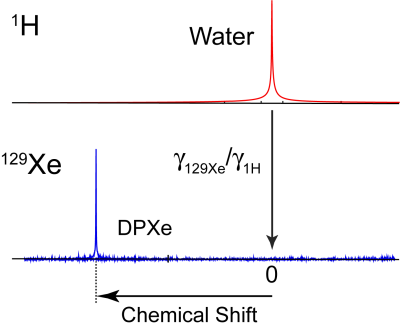
The proton-based reference method utilizes the water signal, originating from the same region of interest (ROI) from which the DPXe signal is acquired, to establish a 0-ppm 129Xe reference, similar to what was previously done by Zhang, et al.7 Specifically, after the acquisition of a proton spectrum, the water resonance frequency is measured and scaled by the 1H and 129Xe gyromagnetic ratios to obtain the absolute 0 ppm 129Xe frequency (Fig. 1). The DPXe CS is then measured with respect to this frequency. This method produces CS values that are typically 2-4 ppm higher than those obtained by referencing the DPXe to the 129Xe gas phase, depending on the tissue analyzed. In vivo experiments were performed on a total of 4 male Fisher rats on a 9.4 T Bruker BioSpec 94/30 system. Rats were placed into a volume 1H coil, with a 129Xe surface coil (1 cm diameter) located above the ROI (head, liver, kidney, and lower leg). For the acquisition of 129Xe spectra, anesthetized animals were ventilated with 75-vol% hyperpolarized 129Xe gas and 25-vol% oxygen. In vitro experiments were performed on a 500 MHz Varian Inova NMR spectrometer on freshly excised samples of adipose tissue (excised from rats and humans), muscle (excised from rats), cerebrospinal fluid (CSF, pooled from humans; Lee Biosolutions Inc., Maryland Heights, MO, USA), and several oils and triglycerides. For the experiments, samples were placed in a high-pressure NMR tube and connected to a vacuum-pump system. A freeze-thaw cycle was repeated 3 times to completely degas the sample. Then the sample was placed under 1 atmosphere of 86% isotopically enriched 129Xe gas and allowed to equilibrate for 24 hours. 1H and 129Xe spectra were then collected to establish the CS of xenon dissolved in the tissue or liquid of interest.Results and Discussion
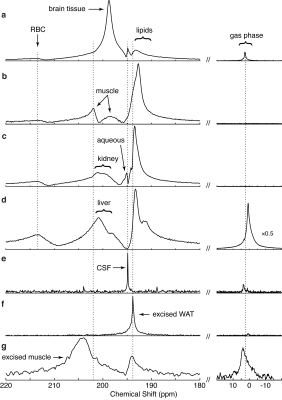
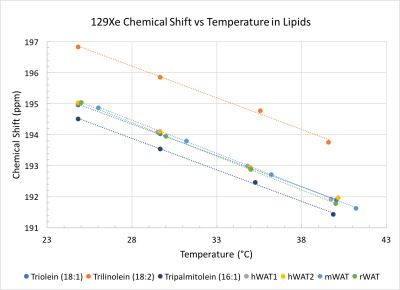
The proton-based method resulted in consistent CS values for a variety of organs and tissues in rats where gas-phase referencing presented variations of up to 4 ppm. In addition, CS values obtained in vitro nicely matched those obtained in vivo, enabling the correct re-assignment of some of the resonance frequencies previously observed in vivo (Fig. 2). Specifically, when CS values obtained in vitro from excised muscle, white adipose tissue (WAT) and CSF were compared to those obtained in vivo, we were able to confidently assign in vivo peaks at 193 ppm (188-189 ppm from the gas phase) to lipids (31°C), 194.9 ppm (~192 ppm from the gas phase) to CSF, 198.7 ppm to brain tissue, 198.8 ppm to muscular tissue and plasma, 202.5 ppm to muscular tissue, and 213-214 ppm to red blood cells, as shown in Figure 3. Similarly, when the CS of xenon dissolved in lipids was referenced to that of nearby methylene protons, no significant differences were seen in CS values or in the DPXe CS temperature dependence between rat, mouse and human adipose tissue, which displays an average linear temperature coefficient of -0.204 ppm/°C and offset of 200.11 ppm, while differences in CS values were seen between adipose tissue and oils. Specifically, comparison of DPXe CS spectra of neat tripalmitolein (16:1), triolein (18:1) and trilinolein (18:2) show that the DPXe CS offset is sensitive to triglyceride saturation, whereas the temperature coefficient is not (Fig. 4).Conclusion
Macroscopic susceptibility gradients produce large shifts in the apparent CS of DPXe resonances and prevent correct identification and assignment of peaks. By referencing the DPXe CS to that of nearby water or lipid protons, we can remove the effect of macroscopic and microscopic susceptibility gradients and obtain consistent CS values for DPXe signals originating from the same organ or tissue compartments, as well as enable calibration of absolute temperature measurements using DPXe in humans in vivo.Acknowledgements
This work was supported by NIH grant fund R01 DK108231.References
1. Goodson, B. M. Nuclear magnetic resonance of laser-polarized noble gases in molecules, materials, and organisms. J. Magn. Reson. 155, 157–216 (2002).
2. Rao, M., Stewart, N. J., Norquay, G., Griffiths, P. D. & Wild, J. M. High resolution spectroscopy and chemical shift imaging of hyperpolarized 129Xe dissolved in the human brain in vivo at 1.5 tesla. Magn. Reson. Med. 75, 2227–2234 (2016).
3. Norquay, G. et al. Relaxation and exchange dynamics of hyperpolarized 129Xe in human blood. Magn. Reson. Med. 74, 303–311 (2015).
4. Branca, R. T., Zhang, L., Burant, A., Katz, L. & McCallister, A. A Preliminary Study on Brown Adipose Tissue Detection Using PET/MR. in Proceedings of the International Society of Magnetic Resonance in Medicine 1054 (2016).
5. Miller, G. W. et al. Dynamic Spectroscopy of Dissolved-Phase Xenon-129 in the Human Kidney. in Proceedings of the International Society of Magnetic Resonance in Medicine 1182 (2017).
6. Burant, A., Zhang, L., McCallister, A. & Branca, R. T. In Vivo Temperature Imaging Using Lipid-dissolved Hyperpolarized Xenon-129. in Proceedings of 56th Experimental Nuclear Magnetic Resonance Conference 87 (2015).
7. Zhang, L. et al. Accurate MR thermometry by hyperpolarized 129Xe. Magn. Reson. Med. 78, 1070–1079 (2017).
Figures
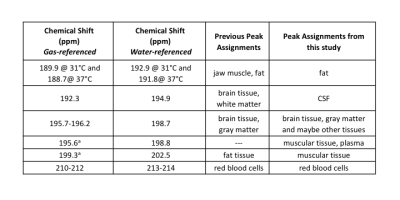
Summary of the CS of the major peaks observed in this study and their assignments. Chemical shift values are reported with respect to the gas phase and to our water-based reference. Included is a summary of previous peak assignments.
a Since no gas-phase 129Xe signal was present in the in vivo muscle spectrum, these gas-referenced values were estimated by taking the CS difference between the water-referenced DPXe CS values for these peaks and the primary brain tissue peak, and adding the gas-referenced DPXe CS value for gray matter as reported in Rao et al.2