2475
Microporous Lung Phantoms for 19F-MRI of Inhaled Imaging Agents with Physiologically Representative Relaxation Times1Newcastle Magnetic Resonance Centre, Newcastle University, Newcastle upon Tyne, United Kingdom
Synopsis
A primary characteristic of 19F-MRI of pulmonary ventilation is the short in vivo T2* of the inhaled imaging agent caused by the inhomogeneous magnetic environment proximal to the alveolar walls. This study describes two novel methods for fabrication of phantoms that mimic the physical and magnetic properties of alveolar tissue. In both cases the perfluorinated gas phase imaging agent is suspended in a stable microporous foam medium. The fabrication techniques permitted precise control of either bubble size or gas/liquid ratio. Highly monodisperse stable foams were formed with a perfluoropropane T2* of 2ms, comparable to that measured in the human lung.
Introduction
19F-MRI of pulmonary ventilation utilises an inhaled imaging agent such as a 79%:21% perfluoropropane/oxygen gas mixture (PFP/O2).1,2 The pulmonary alveolar structure produces magnetic susceptibility gradients between the diamagnetic parenchyma and the paramagnetic oxygen in inhaled PFP/O2. This reduces the PFP T2* from 12ms in a magnetically homogeneous environment to T2* = 2.2 ms in the human lung.3 A lung-representative phantom that reflects the magnetic environment of human alveoli would permit directly translatable acquisition development without necessitating human subjects. Literature on the subject is scarce, with sponge-based phantoms being most widely used.4,5 However, due to limitations with sourcing standardised materials, control over physical and magnetic properties is minimal. The purpose of this study was to develop stable microporous foams of known pore size, susceptibility gradients, and gas/liquid ratio, that exhibit a PFP T2* close to that measured in the human lung. Two novel foam fabrication methods are presented, yielding monodisperse (uniform bubble diameter) and polydisperse (heterogeneous bubble diameter) phantoms.Methods
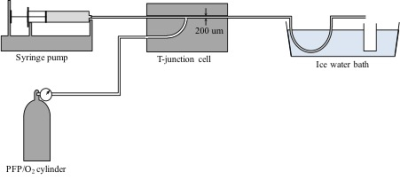
Monodisperse foams were produced by mixing an aqueous detergent solution with PFP/O2 gas mixture in a 200 μm diameter T-junction microfluidic chip (Dolomite, UK). The detergent solution comprised 2 wt% PEG-40 stearate and 1 wt% low melting point agarose in water. Gas pressure and detergent solution flow rate were varied to alter foam bubble size and water content, and the resultant foam was collected in a chilled vial to allow agarose gelling, providing foam structural stability. Figure 1 shows a schematic of the foam production apparatus. Polydisperse foams were produced by mixing an aqueous solution of ovalbumin, citric acid and triethyl citrate (Dr Oetker egg white powder) in a 1:4 ratio with PFP/O2 gas, and repeatedly passing the mixture through a ~1 mm diameter tube to form a homogeneous foam. Physical and magnetic properties of both foam types were assessed. Foam bubble diameter was measured by photomicrography from 100 bubbles per sample, and intra- and inter-sample variability determined. The magnetic susceptibility of the liquid and gas components of the foams were measured on a susceptibility balance (Sherwood Scientific, UK). Perfluoropropane –CF3 T1, T2 and T2* were measured using a 2.5 cm diameter 19F solenoid coil interfaced to a Philips 3.0 T scanner. Photomicrography was repeated 1 hour after fabrication (after the MR acquisitions) in order to identify change in bubble size as a measure of foam stability.Results
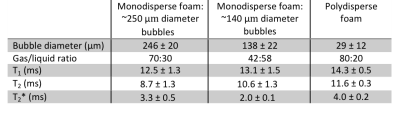
The liquid components of both foam types were found to have a magnetic susceptibility equal to that of water. Figure 2 shows photomicrographs of monodisperse foam with two distinct bubble sizes (mean diameters = 246±20 μm and 138±22 μm respectively), and a photomicrograph of polydisperse foam (mean diameter = 29±12 μm). No significant change in bubble size was measured in any of the samples after one hour. The water content of the three foams was 30%, 58% and 20% respectively, and the corresponding perfluoropropane –CF3 T2* was 3.3±0.5 ms, 2.0±0.1 ms and 4.0±0.2 ms respectively. A summary of the physical and relaxation properties measured is listed in Table 1.Discussion
The monodisperse foam fabrication method produced a highly stable foam with lifetime considerably longer than the duration of MR experiments. The bubble sizes formed were similar in size to human alveoli, with PFP T2* representative of in vivo values and increased with bubble size, reflecting the reduced influence of the susceptibility gradients. Fine control of gas/liquid ratio was possible only for polydisperse foams, which provided an economical and fast fabrication process. Although only tested with PFP, it is expected that these phantoms will be equally valuable for protocol development on other perfluorinated imaging agents and thermally polarised 129Xe.Conclusion
Two lung-representative phantom fabrication techniques were described. Both reflect physical characteristics of human pulmonary alveoli and provide novel tools for the development of quantitative MR imaging of pulmonary ventilation.Acknowledgements
References
1: Halaweish, A.F. and Charles, H.C. (2014) 'Physiorack: An integrated MRI safe/conditional, Gas delivery, respiratory gating, and subject monitoring solution for structural and functional assessments of pulmonary function', Journal of Magnetic Resonance Imaging, 39(3), pp. 735- 741.
2: Gutberlet, M., Kaireit, T.F., Voskrebenzev, A., et al. (2017) ‘Free-breathing dynamic 19F gas MR imaging for mapping of regional lung ventilation in patients with COPD’, Radiology, 0(0) pp. 1-12.
3: Couch, M.J., Ball, I.K., Li, T., et al. (2013) 'Pulmonary ultrashort echo time F-19 MR imaging with inhaled fluorinated gas mixtures in healthy volunteers: Feasibility', Radiology, 269(3), pp. 903-909.
4: Menke, J., Helms, G. and Larsen, J. (2010) 'Viewing the effective k-space coverage of MR images: phantom experiments with fast Fourier transform', Magnetic Resonance Imaging, 28(1), pp. 87-94.
5: Molinari, F., Madhuranthakam, A.J., Lenkinski, R., et al. (2014) 'Ultrashort echo time MRI of pulmonary water content: assessment in a sponge phantom at 1.5 and 3.0 Tesla', Diagnostic and Interventional Radiology, 20(1), pp. 34-41.
Figures