2474
Accelerated 19F-MR Imaging of Inhaled Perfluoropropane for Assessment of Pulmonary Ventilation1Newcastle Magnetic Resonance Centre, Newcastle University, Newcastle upon Tyne, United Kingdom
Synopsis
MRI of inhaled perfluoropropane offers a safely repeatable modality for mapping pulmonary ventilation. However, as a thermally polarised gas, signal is scarce and acquisitions are limited to breath hold durations or require respiratory gating. Improving the temporal resolution would present the opportunity to implement dynamic imaging or improve image quality in breath hold acquisitions. In this study, the acquisition time was reduced by partially sampling k-space using a compressed sensing technique. A 3-fold decrease in acquisition time was achieved whilst maintaining visually similar image quality. An average SNR of 25:1 was measured in a 6s 3D acquisition in healthy volunteers.
Introduction
MRI of exogenous imaging agents offers a safely repeatable modality to assess regional pulmonary ventilation. Notable advancements have been made in hyperpolarised noble gas imaging, with the technique conducive to achieving high SNR images.1,2 Recent studies have validated the potential utility of imaging inhaled perfluoropropane (C3F8, PFP).3,4 MRI of thermally polarised PFP has comparably scarce signal, but benefits from requiring less technical preparation than hyperpolarised gas imaging and can exploit the short longitudinal relaxation time of PFP to allow a high degree of signal averaging. However, the strict breath-hold or dynamic length acquisition time restrictions present an additional challenge. Compressed sensing (CS) partially samples the k-space domain pseudo-randomly, reducing the acquisition time correspondingly.5 We aimed to maximise SNR by implementing a previously optimised 3D spoiled gradient echo (SPGR) sequence, and improve acquisition speed by applying compressed sensing techniques to image inhaled PFP in healthy volunteers, offering potential future utility in patients with respiratory disease.Methods
This study was approved by the local NHS Research Ethics Committee (Ref 14/NE/0135). A 3D SPGR acquisition (TE = 1.7 ms, TR = 7.5 ms, flip angle = 50°, FOV = 400 x 320 x 250 mm3, resolution = 10 x 10 x 10 mm3, bandwidth = 500 Hz/pixel, B1 = 4 uT, averages = 4) was used, resulting in an 18 s acquisition time. A compressed sensing sampling pattern was subsequently applied, offering a 3-fold acceleration, reducing the acquisition time to 6 s. The utility of the accelerated acquisition sequence was tested in three healthy volunteers during a breath hold following three deep wash-in inhalations of a 79% PFP/21% O2 gas mixture. The same inhalation and breath hold protocol was then repeated on each volunteer using the fully sampled acquisition protocol. Images were acquired using a 50 cm long quadrature birdcage coil (Rapid Biomedical) interfaced to a Philips Achieva 3.0 T system. In each of the images, SNR was calculated from a 50 x 50 mm2 ROI in the apex of the right lung. A threshold was then applied to each image at a value of three standard deviations below the mean signal, permitting calculation of ventilated volume and comparison of the two acquisition techniques.Results
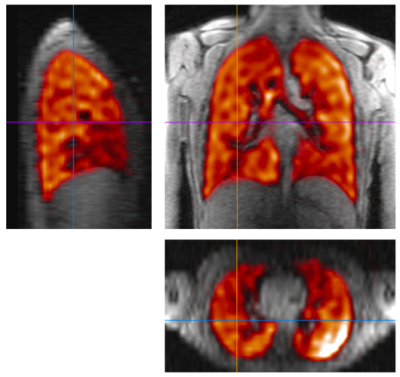
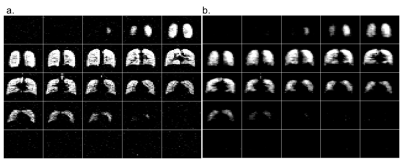
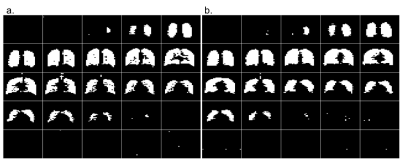
Figure 1 depicts slices through the fully-sampled 19F 3D SPGR acquisition overlaid on a 1H proton anatomical scan. Figures 2a and 2b depict the 25 coronal slices through a fully sampled image and the CS image respectively. The 18 s fully sampled acquisition sequence achieved an average SNR of 20±4. The same acquisition with CS achieved an average SNR of 25±4 in 6 s. Using the described thresholding technique, the ventilated volume of the CS images was calculated from masked images (Figure 3) and found to deviate from the fully-sampled results by 4.8±1.4%. Scans performed with a single average rather than n=4 produce full 3D images with SNR >10:1 from a 1.5 second scan.Discussion
The in vivo comparison of the fully sampled and the 3× accelerated acquisition protocols produced visually similar images in three healthy volunteers, with ventilated volume values within 5% using a simple thresholding technique. The time saved can be used to acquire data at a finer resolution during breath hold acquisition, or to collect more signal averages to improve the SNR. These preliminary results also demonstrate acquisitions with a minimum temporal resolution of 1.5 s, which offers the potential for dynamic imaging studies mapping wash-in and wash-out of imaging agents in patients with obstructive lung disease. Future studies will optimise the sampling pattern and degree of acceleration for ventilation images in patients with respiratory disease and test the utility of this accelerated imaging technique for detecting ventilation defects.Conclusion
We have demonstrated that 3D full lung images can be acquired within a 6 s breath hold by application of an optimised SPGR imaging protocol and compressed sensing techniques, thus broadening the potential utility of this imaging technique.Acknowledgements
No acknowledgement found.References
1. Wild, J., Smith, L., Horn, F., Collier, G., Swift, A., Stewart, N., Rao, M., Norquay, G., Hughes, D., Ugonna, K. and Marshall, H. (2015) 'Hyperpolarised gas MR lung imaging – Breaks through to clinical practice', European Respiratory Journal, 46(59).
2. Yablonskiy, D., Sukstanskii, A. and Quirk, J. (2015) 'Diffusion lung imaging with hyperpolarized gas MRI', NMR in Biomedicine. 30(3), pp.1-24.
3. Couch, M.J., Ball, I.K., Li, T., Fox, M.S., Littlefield, S.L., Biman, B. and Albert, M.S. (2013) 'Pulmonary ultrashort echo time F-19 MR imaging with inhaled fluorinated gas mixtures in healthy volunteers: Feasibility', Radiology, 269(3), pp. 903-909.
4. Halaweish, A.F., Moon, R.E., Foster, W.M., Soher, B.J., McAdams, H.P., MacFall, J.R., Ainslie, M.D., MacIntyre, N.R. and Charles, H.C. (2013) 'Perfluoropropane gas as a magnetic resonance lung imaging contrast agent in humans', Chest, 144(4), pp. 1300-1310.
5. Mann LW, Higgins, DM, Peters, CN, et al., (2015) ‘Accelerating MR imaging liver steatosis measurement using combined compressed sensing and parallel imaging: A quantitative evaluation’, Radiology, 278(1) pp. 247-256.
Figures