2472
Combination of Perfluoropropane and oxygen-enhanced MRI-derived washout kinetics for detection of ischemic injury to lungs in a porcine ex-vivo perfusion systemJulius Renne1,2, Marcel Gutberlet1,2, Andreas Voskrebenzev1,2, Agilo Kern1,2, Till Kaireit1,2, Jan Bernd Hinrichs1,2, Peter Braubach3, Christiane S Falk2,4, Klaus Höffler5, Gregor Warnecke2,5, Axel Haverich5, Frank Wacker1, Jens Vogel-Claussen1,2, and Norman Zinne2,5
1Diagnostic and Interventional Radiology, Hannover Medical School, Hannover, Germany, 2Integrated Research and Treatment Center Transplantation (IfB-Tx), Hannover, Germany, 3Institute for Pathology, Hannover Medical School, Hannover, Germany, 4Institute for Transplant Immunology, Hannover Medical School, Hannover, Germany, 5Clinic for Cardiothoracic and Transplantation Surgery, Hannover Medical School, Hannover, Germany
Synopsis
Ex-vivo lung perfusion and ventilation systems are a promising new tool for conditioning marginal lung allografts. However, reliable biomarkers for evaluating graft function are missing. In this study MRI-derived fluorine and oxygen washout times are to be evaluated as lung function parameters in a porcine model of ischemia. Washout time for oxygen is prolonged while fluorine washout is not in lungs after warm ischemia compared to normal controls, which might reflect pulmonary edema limiting oxygen diffusion. Determination of fluorine and oxygen washout is feasible in an ex-vivo lung perfusion system and seems to be promising tools for evaluating graft function.
Introduction
Ex-vivo perfusion systems for pulmonary allografts prior to transplantation are a promising new tool for conditioning of marginal organs. However, reliable parameters for graft function within the system are missing. Washout kinetics of oxygen and fluorinated gases have been introduced to quantify regional lung ventilation and therefore to give local information about lung function. In this proof of concept study the feasibility of using oxygen as well as perfluoropropane washout time in an ex-vivo lung perfusion system was tested. Furthermore, the ability of detecting pathologic changes due to ischemic lung injury was tested for both methods.Methods
This study was approved by the federal animal welfare committee. Twelve female pigs of German Landrace were anesthetized. Pulmonary and bronchial arteries of the left lung were clamped to induce ischemic injury (76 to 199 min, median 170 min), right lungs remained perfused as controls. After cold flush with Perfadex solution (XVIVO Perfusion AB), lungs were installed in a modified ex-vivo lung perfusion system container (OCS lung system, TransMedics; Figure 1) using full blood and Steen solution (XVIVO Perfusion AB) with a perfusate flow of 1.5 l/min and a respiration rate of 10/min with 500 ml tidal volume. After allowing a minimum wash-in time of 5 minutes for both 100% oxygen or a mixture of perfluoropropane and oxygen the respirator was switched to normal room air. Magnetic resonance imaging was performed on a 1.5 tesla scanner (Avanto, Siemens) using a standard spine coil in combination with body matrices for proton imaging and a dedicated 19F-coil (Rapid Biomedical) tuned to 59.9 MHz (Helmholtz transmit coil and a 16 channel receive coil) for imaging of perfluoropropane. Oxygen washout dynamics were measured using an Inversion-Recovery HASTE sequence (TI=1100 ms, TE=11 ms, TR=6000ms, flip angle=180°, FOV=45 x 45 cm2, matrix size=128 x 128, slice thickness=15 mm) on a central coronal slice. 19F wash-out dynamics were measured with a 3D spoiled gradient echo sequence (TE=5.1 ms, TR=12 ms, flip angle=50°, FOV=50 x 50 cm2, matrix size=64 x 64, slice thickness=40 mm, GRAPPA imaging with acceleration factor of 2 in A/P direction). One central coronal slice corresponding to the oxygen washout image was analyzed. Washout times win were estimated voxelwise by mono-exponential fitting of the temporal signal. At the end of the EVLP run samples from both lungs were collected and weight. After drying in an oven for 3 days samples were weighed again to assess the wet / dry weight ratio as a marker for edema formation. Statistical analysis was performed using the Wilcoxon matched pairs test. Results are given as median [interquartile range].Results
No ventilation defect was seen on the 19F images prior to washout (Figure 2a). The washout time for 19F did not differ between lungs after warm ischemia (67.9 s [54.6 s; 84.6 s]) compared to normal lungs (69.8 s [54.9 s; 79.3 s]) (p=0.97; Figure 2b). The oxygen washout time differed significantly between lungs with and without ischemia (p=0.019). The ischemic lungs showed a prolonged washout time of 44.6 s [43.1 s; 54.7 s] compared to 37.3s [31.2 s; 47.2 s] at an equal breathing rate of 10/min (Figure 2c). Wet / dry ratio of ischemic lungs was significantly higher than in normal control lungs (4.76 [4.55 ; 5.45] vs 4.53 [4.16 ; 4.74], p=.024).Discussion
Ischemia-reperfusion injury is known to induce an increase in pulmonary vascular permeability and therefore causing pulmonary edema1,2. The observed wet / dry ratio difference shows the induced edema in ischemic lungs. This edema leads to a thickening of the alveolar walls and as a consequence, to a reduced oxygen diffusion over the basal membrane. Therefore, the observed prolonged washout times for oxygen may reflect this pathophysiologic pattern. As inert fluorinated gases have a low solubility in blood3, fluorine washout primarily reflects the ventilation of the lung, whereas oxygen washout time is a combination of ventilation, perfusion and oxygen diffusion capacity. Therefore, the combination of fluorine with oxygen washout seems to be a promising new tool in pulmonary magnetic resonance imaging, as this might deliver a marker for oxygen diffusion via the membrane.Conclusion
The combination of oxygen and fluorine washout kinetics might be a promising new marker for graft function and should be evaluated in further studies comparing this parameter with graft function and clinical outcome following transplantation. Furthermore, this combination should be evaluated as a marker for oxygen diffusion in other pulmonary diseases.Acknowledgements
No acknowledgement found.References
- Allison RC, Kyle J, Adkins WK, et al. Effect of ischemia reperfusion or hypoxia reoxygenation on lung vascular permeability and resistance. J. Appl. Physiol. 1990;69(2):597–603.
- de Perrot M, Liu M, Waddell TK, Keshavjee S. Ischemia–Reperfusion–induced Lung Injury. Am J Respir Crit Care Med. 2003;167(4):490–511.
- Wagner PD, Saltzman HA, West JB. Measurement of continuous distributions of ventilation-perfusion ratios: theory. J Appl Physiol 1974; 36: 588 – 599.
Figures
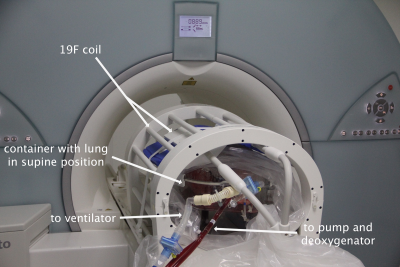
Figure 1: Modified ex-vivo lung perfusion
system containing a pig lung in supine position in the MRI-suite.
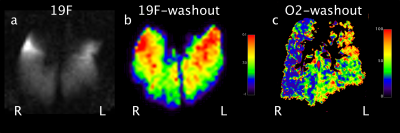
Figure 2: a) 19F gradient echo image
before start of washout, showing no ventilation defects in both lungs b) 19F
washout map without any difference in washout times (s) for ischemic (left) and normal (right) lungs. c) Oxygen washout map with increased washout times (s) in the left lung.