2469
Dynamic contrast-enhanced MRI in the lung – evaluation of measures of pulmonary oedema and pulmonary endothelial permeability in healthy subjects and patients with chronic heart failure1GSK, Stevenage, United Kingdom, 2GSK Clinical Unit, Cambridge, United Kingdom, 3Experimental Medicine & Immunotherapeutics, University of Cambridge, Cambridge, United Kingdom, 4Cambridge University Hospitals, Cambridge, United Kingdom, 5Bioxydyn Ltd, Manchester, United Kingdom, 6GSK, Collegeville, PA, United States, 7GSK, King of Prussia, PA, United States, 8Centre for Imaging Sciences, The University of Manchester, Manchester, United Kingdom
Synopsis
MRI has previously demonstrated increased lung water content in patients with heart failure (HF), but has not yet been used to distinguish between intravascular and extravascular water in these patients. This study evaluated dynamic contrast-enhanced MRI (DCE-MRI) for measuring pulmonary oedema and endothelial permeability in healthy volunteers (HV) and chronic HF patients at rest and post-exercise. DCE-MRI showed a redistribution of lung water towards the interstitial space in chronic HF, as compared to HV, suggesting this method may have value as a novel endpoint for dose-ranging and proof-of-mechanism studies in chronic HF. No exercise-induced change was seen in either group.
Introduction
MRI has potential for quantifying pulmonary oedema, and has demonstrated increased lung water content in patients with heart failure (HF)1. However, quantification remains problematic, and it is not possible to distinguish between intravascular and extravascular water using existing techniques. Dynamic contrast-enhanced (DCE) MRI is an established technique enabling quantification of the respective fraction of fluid volume in the plasma (vp) versus the fraction of fluid volume in the interstitial space (ve) as well as the contrast agent leakage (Ktrans – dependent on endothelial permeability). The aim of this Phase 1 methods validation study was to evaluate DCE-MRI for measuring pulmonary oedema and endothelial permeability in healthy volunteers (HV) and chronic HF. We hypothesised sensitivity of ve and Ktrans to increased pulmonary oedema and endothelial permeability (respectively) in HF, and that both may be affected by exercise.Methods
37 subjects were enrolled in the study (23 HV and 14 chronic HF patients). The evaluable population was defined as patients with MRI data and age >40 yrs, leaving 29 subjects – 17 HV (15 male, 2 female; mean(SD) age = 61.2 (11.4) yrs; mean(SD) body mass index = 26.4 (2.9) kg/m2) and 12 HF patients (10 male, 2 female; mean(SD) age = 67.8 (13.4) yrs; mean(SD) body mass index = 29.2 (2.9) kg/m2). Chronic HF patients had a diagnosis of mild or moderate HF of any aetiology with symptoms corresponding to New York Heart Association (NYHA) Class II or III.
Subjects had three whole-chest DCE-MRI scans each – a baseline scan (Session 1), a repeat baseline ~1 week later for evaluation of repeatability (Session 2), and a post-exercise challenge scan (within 1-3 days of Session 2) to explore effect of exercise on DCE-MRI parameters (Session 3). Imaging was performed on a 1.5 T GE MR450 scanner (software version DV24), using an 8-channel cardiac coil, with subjects in a supine position and free-breathing throughout. The DCE-MRI protocol included variable flip angle (FA) T1-mapping acquisitions, using a 3D coronal spoiled gradient echo sequence with core parameters of: TR/TE = 2.03/0.83 ms, FOV (in plane) = 450 mm x 450 mm, slice thickness = 10 mm and number of slices = 18 (interpolated to 28 slices at 5 mm), acquisition matrix = 112x88, FA = 4/2/7/10° (10 repeated volumes). This was followed by a 7-minute dynamic series (parameters as for T1-mapping, FA = 10°, 170 repeated volumes, temporal resolution = 2.5 s/volume). Intravenous contrast agent was injected at the 15th volume (half-dose Gadovist administered by power injector at 1.5 ml/s, with 25 ml saline flush). Total scan time was ~10 mins, allowing for inclusion of patients with mild orthopnoea.
Data were quality controlled, and breathing motion corrected using a non-linear image registration2. Lungs were segmented from the thorax using a semi-automatic thresholding technique. DCE-MRI analysis was then performed with pharmacokinetic modelling using the extended Tofts model3 to quantify ve, vp and Ktrans on a voxel-wise basis. Analysis allowed production of 3D maps of DCE-MRI parameters in the lung from which summary statistics could be extracted. Data were fitted using a repeat measure analysis of variance (ANOVA) model with terms including patient population (HF or HV), visit, interaction of patient population and visit, with subject ID as block of the repeat factor.
Results
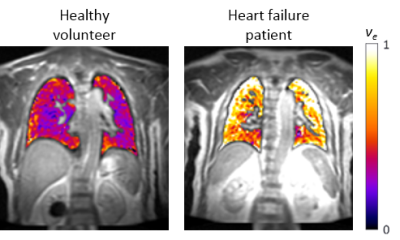
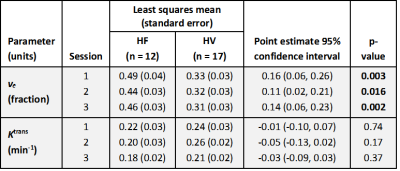
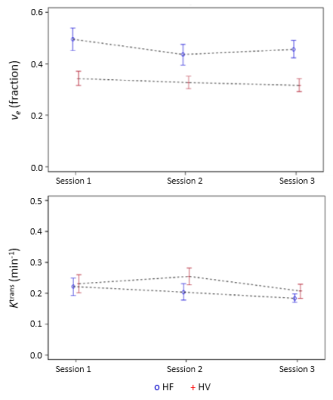
Study procedures were tolerated well by both groups. Increased ve was observed in HF at each scan (Figure 1), with clear separation between HF and HV subjects (Table 1, Figure 2). Analysis of Ktrans, vp, T1 and proton density in the lungs showed no clear difference between HF and HV subjects at each scan. Good reproducibility was demonstrated in measurements of ve and Ktrans between Session 1 and 2 – no statistically significant variability for either HV or HF subjects, and magnitude of the differences observed between the two sessions were generally small. Analysis of DCE-MRI data pre-/post-exercise showed no clear effect of exercise on DCE-MRI parameters for either group (e.g. ve (Session1 vs. Session3), p=0.48 (HV) and p=0.23 (HF)).
Discussion
The greater fraction of fluid volume in the interstitial space, ve, suggests a redistribution of lung water into the interstitial space in chronic HF, as compared to HV. No evidence of differences in endothelial permeability via Ktrans measurement was apparent. A lack of exercise-induced change may reflect difficulty in post-exercise testing standardisation, but could also indicate a more complicated mechanism of change in lung water content post-exercise, or changes in non-MR visible compartments.Conclusion
The results suggest DCE-MRI may have value as a novel endpoint for dose-ranging and proof-of-mechanism studies in chronic HF patients.Acknowledgements
With thanks to the patients, volunteers and staff from GSK Clinical Unit Cambridge and the MRI, Lung and Experimental Medicine departments at Cambridge University Hospitals and University of Cambridge. Study NCT02135861 funded by GSK.References
- Chow K, Toma M, Esch B, et al. Comparison of MRI-derived pulmonary edema measures with LVEDP and serum BNP. J Cardiovasc Magn Reson 2009;11(Suppl 1):P41
- ANTs medical image registration and segmentation toolkit: http://stnava.github.io/ANTs
- Tofts PS, Brix G, Buckley DL, et al. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging 1999;10(3), 223–232
Figures