2467
Contributions of Large Versus Small Airways to MRI Ventilation Heterogeneity in Asthmatics1Robarts Research Institute, London, ON, Canada, 2Medical Biophysics, Western University, London, ON, Canada, 3Medicine, McMaster University, Hamilton, ON, Canada, 4Medicine, Division of Respirology, Western University, London, ON, Canada
Synopsis
Pulmonary functional MRI identifies the exact location of functional abnormalities within the asthmatic lung, however the relative contributions of large and small airways to ventilation heterogeneity in a given patient are unknown. Here, we differentiated hyperpolarized noble gas MRI ventilation into regions corresponding to the large and small airways using patient-specific airway trees and calculated the ventilation defect percent (VDP) related to large and small airways independently. The classification of small and large airway VDP may help with clinical treatment decisions for individualized therapies.
Introduction
Pulmonary functional MRI identifies the exact location of functional abnormalities within the asthmatic lung, and ventilation heterogeneity is now recognized as a hallmark of asthma.1 Such ventilation heterogeneity may serve as an important target for novel asthma therapies, but the particular airways that are abnormal and that relate to specific ventilation defects are not easily discerned. Wedge-shaped ventilation defects have been identified that are spatially-related to abnormally remodeled large central airways measured using computed tomography (CT),2 and another study indirectly showed that ventilation defects were also spatially-related to regions of small airway obstruction measured as air trapping on CT.3 The relative contribution of large or central versus small or peripheral/distal airway obstruction to ventilation heterogeneity in a given patient however, is not yet understood. Thus, the objective of this study was to differentiate the contributions of central and peripheral airways to ventilation heterogeneity using CT airway tree geometries from individual patients.Methods
Subjects and Image Acquisition: In a proof-of-concept exploration, we evaluated baseline hyperpolarized 3He static-ventilation MRI in five patients with a clinical diagnosis of asthma who provided written informed consent to an ethics-board-approved protocol (NCT02351141). All subjects underwent 1H and 3He MRI using a whole-body 3.0T Discovery MR750 system (General Electric Healthcare, USA) with broadband imaging capabilities. For both 1H and 3He MRI, subjects were instructed to inhale a gas mixture from a 1.0L Tedlar bag from functional residual capacity (FRC) and image acquisition was performed during a 16s breath-hold. Anatomical 1H MRI was performed before 3He using the whole-body radiofrequency coil and 1H fast-spoiled, gradient-recalled echo (FGRE) sequence with a partial echo (16s total acquisition time, repetition time (TR)/echo time (TE)/flip angle=4.7ms/1.2ms/30°, field-of-view (FOV)=40x40cm, bandwidth=24.4kHz, matrix=128x80, 15-17 slices, 15mm slice thickness, zero gap). 3He static ventilation images were acquired directly after using a single-channel, rigid elliptical transmit/receive chest coil and fast-gradient echo method with a partial echo (14s total acquisition time, TR/TE/flip angle=4.3ms/1.4ms/7°, FOV=40x40cm, bandwidth=48.8kHz, matrix=128x80, 15-17 slices, 15mm slice thickness, zero gap). All subjects also underwent a single thoracic CT within ten minutes following MRI using a 64-slice Lightspeed VCT system (GEHC) volume-matched to MRI as previously described.4
Image Analysis: 3He static ventilation images were segmented as previously described.5 Airway trees were segmented up to the 7th or 8th generation from thoracic CT images using Pulmonary Workstation 2.0 (VIDA Diagnostics Inc., USA); airways that could be segmented were considered central or large airways, and those that could not be segmented were considered peripheral or small airways. MRI static ventilation images for each patient were deformably co-registered to the thoracic CT volume and airway tree using the modality-independent neighbourhood descriptor (MIND) method6 in MATLAB R2015b (Mathworks, USA). The trachea was manually removed from the airway tree and the remaining airways were dilated on a voxel-wise basis using a 20x3-voxel ellipsoid in order to account for the volume of ventilation supplied by the central airways. This volume was subsequently used to separate the 3He ventilation into the relative contributions of the central and peripheral airways. Whole-lung ventilation defect percent (VDP) was quantified as the ventilation defect volume normalized to the thoracic cavity volume, while VDP of the central and peripheral regions was quantified as the ventilation defect volume in the volume normalized to the volume of the corresponding region. This pipeline is outlined in Figure 1.
Results
We evaluated five asthmatic subjects (3M/2F, 46±7-years) with whole-lung VDP of 7±5% (mean±SD). Figure 2 shows centre-slice static ventilation images as well as central and peripheral regions for all patients with corresponding VDP. For subjects with ventilation defects (whole-lung VDP≥3%, n=4), peripheral VDP was qualitatively greater than central VDP. For the subject without ventilation defects (n=1), peripheral and central VDP were similar. Statistical analysis was not performed for this proof-of-concept evaluation.Discussion
Ventilation defects may serve as important targets for novel asthma therapies. In this proof-of-concept demonstration, we classified 3He ventilation defects based on their relationship to large and small airways using individual patient airway trees. The relative and independent contributions to ventilation heterogeneity from the large and small airways are important to determine for individualized treatment decisions. For example, ultra-fine particle anti-inflammatory inhalers may be more suitable for patients with ventilation heterogeneity related to the small airways as compared to the large airways.Conclusion
Hyperpolarized noble gas MRI ventilation heterogeneity can be differentiated based on the relative contributions from the large and small airways, and this may be important for clinical treatment decision-making.Acknowledgements
No acknowledgement found.References
1 Teague, W. G., Tustison, N. J. & Altes, T. A. Ventilation heterogeneity in asthma. The Journal of asthma : official journal of the Association for the Care of Asthma 51, 677-684, doi:10.3109/02770903.2014.914535 (2014).
2 Svenningsen, S. et al. What are ventilation defects in asthma? Thorax 69, 63-71, doi:10.1136/thoraxjnl-2013-203711 (2014).
3 Fain, S. B. et al. Evaluation of structure-function relationships in asthma using multidetector CT and hyperpolarized He-3 MRI. Academic radiology 15, 753-762, doi:10.1016/j.acra.2007.10.019 (2008).
4 Kirby, M., Pike, D., Coxson, H. O., McCormack, D. G. & Parraga, G. Hyperpolarized (3)He ventilation defects used to predict pulmonary exacerbations in mild to moderate chronic obstructive pulmonary disease. Radiology 273, 887-896, doi:10.1148/radiol.14140161 (2014).
5 Kirby, M. et al. Hyperpolarized 3He magnetic resonance functional imaging semiautomated segmentation. Acad Radiol 19, 141-152, doi:10.1016/j.acra.2011.10.007 (2012).
6 Heinrich, M. P. et al. MIND: modality independent neighbourhood descriptor for multi-modal deformable registration. Medical image analysis 16, 1423-1435, doi:10.1016/j.media.2012.05.008 (2012).
Figures
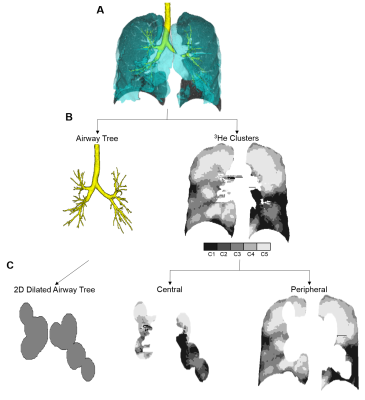
Figure 1. Pipeline for differentiating the contributions of central and peripheral airways to ventilation heterogeneity.
A) 3He static ventilation and CT volume and airway tree deformably co-registered. B) Registered airway tree and 3He clusters (cluster 1 [C1], signal void, to C5, hyperintense signal). C) 2D-slice of airway tree volume dilated on a voxel-wise basis using a 20x3-voxel ellipsoid and corresponding central and peripheral ventilation volumes.
Whole-lung VDP=15%; Central VDP=7%; Peripheral VDP=19%.
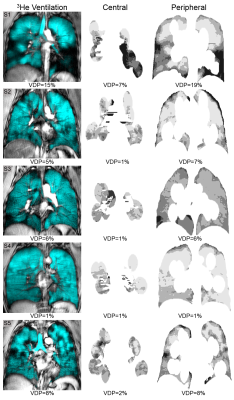
Figure 2. Centre slice 3He static ventilation and corresponding central and peripheral ventilation volumes for five asthmatic subjects.
Centre slice 3He static ventilation (cyan) co-registered to anatomical 1H (grey-scale) and corresponding central and peripheral ventilation volumes.