2465
GRE bSSFP vs. FLASH based Fourier Decomposition lung MRI at 1.5T: evaluation of image quality, fractional ventilation and lung perfusion in healthy volunteers1Institute of Diagnostic and Interventional Radiology, Medical School Hannover, Hannover, Germany, 2German Center for Lung Research, Hannover, Germany
Synopsis
The comparison between different MRI sequences for assessment of lung ventilation and perfusion using phase-resolved functional lung MRI post-processing (PREFUL) needs further evaluation to support clinical translation. Our study compares two gradient echo (GRE) balanced steady state free precession (bSSFP) sequences (one commercially available and one modified by Bauman et al.) and one GRE Fast Low Angle Shot (FLASH) sequence regarding signal-to-noise ratio, fractional ventilation and lung perfusion. In summary, the bSSFP sequence modified by Bauman provides significantly higher SNR values and better perfusion values in the lung parenchyma compared to the commercially available bSSFP and FLASH sequences using PREFUL.
INTRODUCTION
Functional lung MRI with Fourier Decomposition (FD) analysis has become a possibility of contrast-agent-free regional ventilation and perfusion assessment during free breathing with initial promising clinical results1–4. Regarding the MRI sequence, important requirements are high temporal and reasonable spatial resolution as well as sufficient signal in the lung to perform voxel-based evaluation of the cardiac and ventilation cycle in the lung parenchyma simultaneously. Most FD studies use balanced steady-state free-precession (bSSFP) sequence, which is well suited for perfusion imaging due to high T2/T1 ratios of blood in comparison with other tissues5. However, bSSFP sequences are susceptible for banding artifacts due to field nonuniformity6. Bauman et al. optimized the bSSFP sequence for the setting of FD analysis at 1.5T MR system in order to reduce banding artifacts and improve lung SNR1. As an alternative approach the standard spoiled GRE sequence Fast Low Angle Shot (FLASH) has been described7,8. However, to date no direct comparison between different image acquisition methods has been reported. Therefore, the purpose of this study is to compare the standard FLASH, the standard bSSFP and bSSFP sequence modified by Bauman1, regarding image quality, ventilation and perfusion of the lung.METHODS
Seven healthy volunteers (3 female, 4 male, age range: 25 – 39 years) were examined on a 1.5T scanner (Magnetom Avanto, Siemens Healthineers, Erlangen, Germany). A coronal slice of the lungs was acquired using a standard 2D FLASH, a standard bSSFP and the bSSFP sequence modified by Bauman1 of each volunteer. The sequences had the following parameters, 2D FLASH: TE 0.82 ms, TR 3 ms, flip angle 5°, FOV 50 x 50 cm2, slice thickness 15 mm, image matrix 128x128 interpolated to 256 x 256, bandwidth 1500 Hz/pixel, temporal resolution 227 ms per image; standard bSSFP: TE 0.86 ms, TR 2,22 ms, flip angle 35°, FOV 50 x 50 cm2, slice thickness 15 mm, image matrix 128x128 interpolated to 256 x 256, bandwidth 1000 Hz/pixel, temporal resolution 323 ms per image; bSSFP (Bauman): TE 0.64 ms, TR 1.41 ms, flip angle 35°, FOV 50 x 50 cm2, slice thickness 15 mm, image matrix 128x128 interpolated to 256 x 256, bandwidth 2055 Hz/pixel, temporal resolution 318 ms per image. The image registration towards intermediate respiratory position was performed by using Advanced Normalization Tools (ANTS)9. Fractional Ventilation and Perfusions maps were computed by using the PREFUL method as described by Voskrebenzev et al8. Furthermore, computation of the lung perfusion was performed as demonstrated by Kjørstad et al4. The SNR was calculated using the pseudo multiple replica method10. In addition, the calculated signal-to-noise ratio (SNR) of the lung parenchyma for image quality comparison was normalized regarding differences in sequence type, time of acquisition and frequency bandwidth for all sequences.RESULTS
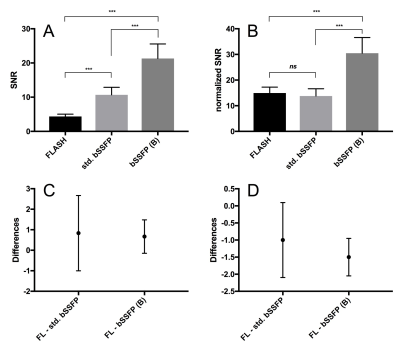
The Friedman test and Dunn’s multiple comparison test were performed as ANOVA analysis of paired SNR, normalized SNR, fractional ventilation and perfusion calculations. Figure 1 summarizes the results regarding the evaluated parameters. The SNR in the lung parenchyma of the bSSFP (Bauman) sequence had significantly higher values (P < 0.001) compared to the FLASH and the standard bSSFP sequences. Also, the standard bSSFP sequence had higher lung parenchyma SNR values compared to FLASH sequence. There was no statistically difference between the FLASH and the standard bSSFP sequence regarding the normalized SNR (P = 0.38). Figure 2 shows exemplarily fractional ventilation and perfusion maps. No statistically significant difference was observed regarding fractional ventilation values between the tested sequences (P = 0.43). The bSSFP (Bauman) sequence showed significantly higher lung/aorta ratios on the calculated perfusion maps (P = 0.02) compared to the other sequences. While notable banding in the lung parenchyma was observed in the commercially available bSSFP sequence, it was absent in the bSSFP Bauman and FLASH sequences (Figure 2).DISCUSSION
The similar fractional ventilation results could be explained by the computation itself (Fractional Ventilation = ΔV/Vinsp), therefore possible SNR differences have no influence. bSSFP sequences are known to generate higher tissue contrast, as demonstrated with higher SNR values in the lung parenchyma, because the signal depends on the T2/T1 ratio. Moreover, regarding the normalized SNR measurements the bSSFP by Bauman outperforms both, the FLASH and the standard bSSFP, clearly. Therefore, the modified bSSFP sequence could provide higher perfusion signal in the lung parenchyma relative to the aorta.CONCLUSSION
The bSSFP (Bauman) sequence could provide improved lung perfusion assessment compared to the tested GRE FLASH sequence. Further research needs to show if this translates into improved depiction of perfusion changes in patients with lung disease.Acknowledgements
This research was fund by the German Center for Lung Research (DZL).References
1. Bauman G, Puderbach M, Deimling M, Jellus V, Chefd’hotel C, Dinkel J, Hintze C, Kauczor HU, Schad LR. Non-contrast-enhanced perfusion and ventilation assessment of the human lung by means of Fourier decomposition in proton MRI. Magn. Reson. Med. 2009;62:656–664. doi: 10.1002/mrm.22031.
2. Schoenfeld C, Cebotari S, Hinrichs J, et al. MR Imaging-derived Regional Pulmonary Parenchymal Perfusion and Cardiac Function for Monitoring Patients with Chronic Thromboembolic Pulmonary Hypertension before and after Pulmonary Endarterectomy. Radiology 2016;279:925–934. doi: 10.1148/radiol.2015150765.
3. Schönfeld C, Cebotari S, Voskrebenzev A, et al. Performance of perfusion-weighted Fourier decomposition MRI for detection of chronic pulmonary emboli. J. Magn. Reson. Imaging 2015;42:72–79. doi: 10.1002/jmri.24764.
4. Kjørstad Å, Corteville DMR, Henzler T, Schmid-Bindert G, Hodneland E, Zöllner FG, Schad LR. Quantitative lung ventilation using Fourier decomposition MRI; comparison and initial study. Magn. Reson. Mater. Physics, Biol. Med. 2014;27:467–476. doi: 10.1007/s10334-014-0432-9.
5. Zapke M, Topf H-G, Zenker M, Kuth R, Deimling M, Kreisler P, Rauh M, Chefd’hotel C, Geiger B, Rupprecht T. Magnetic resonance lung function – a breakthrough for lung imaging and functional assessment? A phantom study and clinical trial. Respir. Res. 2006;7:106. doi: 10.1186/1465-9921-7-106.
6. Graves MJ, Mitchell DG. Body MRI artifacts in clinical practice: A physicist’s and radiologist’s perspective. J. Magn. Reson. Imaging 2013;38:269–287. doi: 10.1002/jmri.24288.
7. Fischer A, Weick S, Ritter CO, Beer M, Wirth C, Hebestreit H, Jakob PM, Hahn D, Bley T, Köstler H. SElf-gated Non-Contrast-Enhanced FUnctional Lung imaging (SENCEFUL) using a quasi-random fast low-angle shot (FLASH) sequence and proton MRI. NMR Biomed. 2014;27:907–917. doi: 10.1002/nbm.3134.
8. Voskrebenzev A, Gutberlet M, Klimeš F, Kaireit TF, Schönfeld C, Rotärmel A, Wacker F, Vogel-Claussen J. Feasibility of quantitative regional ventilation and perfusion mapping with phase-resolved functional lung (PREFUL) MRI in healthy volunteers and COPD, CTEPH, and CF patients. Magn. Reson. Med. 2017;0:1–9. doi: 10.1002/mrm.26893.
9. Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage 2011;54:2033–44. doi: 10.1016/j.neuroimage.2010.09.025.
10. Robson PM, Grant AK, Madhuranthakam AJ, Lattanzi R, Sodickson DK, McKenzie CA. Comprehensive quantification of signal-to-noise ratio and g-factor for image-based and k-space-based parallel imaging reconstructions. Magn. Reson. Med. 2008;60:895–907. doi: 10.1002/mrm.21728.
Figures