2461
Differentiation of Malignant and Benign Pulmonary Lesions with DCE-MR imaging1Radiology, Peking Union Medical College Hospital, Beijing, China, 2Siemens Healthcare, MR Collaborations NE Asia, Beijing, China, 3Siemens Healthcare, MR Collaborations NE Asia, Shanghai, China
Synopsis
The aim of this study was to estimate the diagnostic accuracy of DCE-MR in the differential diagnosis between malignant and benign pulmonary lesions. Thirty patients with suspected lung cancer were recruited. 13 malignancies were proved by pathology. The DCE-MR data was acquired with the TWIST-VIBE technique, and quantitative parameters (Ktrans, Kep, and Ve) were calculated by the Tofts model. Our results demonstrated that malignant lesions had significant higher Ktrans and kep values than benign lesions. The Ktrans and Kep derived from DCE-MR are promising quantification parameters for differentiating lung lesions.
Introduction
Differentiation between benign and malignant lesions non-invasively is of great importance in diagnostic radiology. Currently, computed tomography (CT) remains to be the gold standard. However, CT features, such as shape, edge characteristics etc. are not accurate enough for distinguishing benign from malignant lesions [1, 2]. This is mostly because CT mainly provides morphological characteristics without functional/tissue patterns. Recently, dynamic contrast-enhanced magnetic resonance (DCE-MR) imaging has shown better specificity and accuracy than CT and PET-CT in the differentiation between benign and malignant nodules in several types of oncological diseases [3, 4]. Thus, the purpose of our work was to investigate the diagnostic performance of DCE-MR in the differentiation between malignant and benign lung lesions.Materials and methods
All MR images were acquired on a MAGNETOM Skyra 3T MR scanner (Siemens Healthcare, Erlangen Germany). Patients were selected according to the following criteria: (a) presence of a newly detected solitary pulmonary nodule on CT images, which were solid nodule other than ground-glass opacity nodule and needed further evaluation; (b) absence of calcification or definite fat attenuation of the nodule at CT; (c) nodule diameters larger than 10mm; (d) received anti-inflammatory therapies for at least two weeks, and lesions showing no changes. Finally, 30 patients (13 males, 17 females; age 35-70 years) who fulfilled the inclusion criteria were enrolled in this study.
For the DCE-MRI, gadopentetate dimeglumine (0.1mmol/kg) was administered intravenously at a rate of 2 mL/sec, followed by 20 mL saline. TWIST-VIBE was performed with the parameters set as follows: TR/TE, 3.8/1.23ms; FOV= 360 mm × 300 mm; voxel size = 0.8×0.8×3.0mm3; slice thickness 3mm; number of slices= 56; temporal resolution= 4s; total acquisition time = 8min10s. DCE-MR was obtained upon contrast material injection and was performed with a 10s breath-hold interval between every 12s acquisition, until 480s after contrast material was injected. The Ktrans, Kep, Ve and iAUC were calculated using Tissue4D on a syngo.via workstation (Siemens Healthcare, Erlangen, Germany) and the values derived from DCE-MR were manually measured by drawing a ROI on the lesion. The ability to discriminate malignancies and benignities was analyzed by t-test, and a receiver operating characteristic (ROC) curve analysis was also applied.
Results
In this study, 13 malignancies (2 squamous cell carcinomas and 11 adenocarcinomas) and 17 benign pulmonary lesions were identified. The mean of the longest transverse diameters measured for malignancies was 27 mm (range from 12 to 53 mm), versus 28 mm (range from 11 to 50 mm) for benignities. The averaged values of Ktrans (0.590±1.030/min), Kep (3.920±5.800/min), and iAUC (12.300±11.030) of the malignancies were significantly higher than those of benignities (Ktrans 0.045±0.031min-1, Kep 1.550±2.830/min, iAUC 2.150±4.250) (P<0.05). For Ve, however, no marked difference was found between malignancies and benignities (P>0.05). The results of the ROC analysis showed that the Ktrans had the best diagnostic performance with an area under the curve (AUC) of 0.988 compared with Kep (AUC=0.765) and iAUC (AUC=0.806). The Ktrans value of 0.109/min (sensitivity=90%, and specificity=88.2%) was identified as the best cut-off point by which to differentiate malignancies from benign pulmonary lesions.Discussion
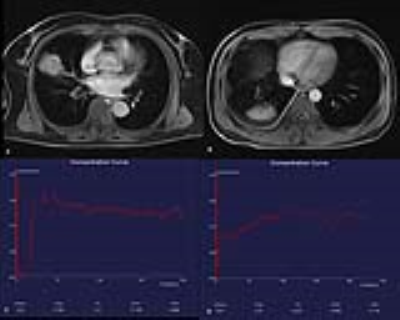
Some previous studies have suggested a potential role of DCE-MRI in differentiating benignities from malignant nodules by classifying the enhancement curve shapes. But semi-quantitative analysis methods may be influenced by properties of the scanners or the injection procedure. According to the curve shape of the tumor wash-in and wash-out, generalized kinetic models were applied to analyze perfusion and permeability of pulmonary lesions. In general, malignant nodules tend to have stronger enhancement with faster upslope, higher maximum peaks, and rapid or gradual washout. The Tofts model used in the present study, which is regarded as a two-compartment model, attempts to account for a vascular plasma space. By comparison with benign lesions, the greater Ktrans and kep values observed in lung lesions can be explained by their higher vascular permeability. Figure 1 shows two cases of pulmonary lesions (1.A,C) of a 60-year-old female with adenocarcinoma in the right middle lobe, Ktrans 3.52, Kep 17.591, Ve 0.2, iAUC 31.11; and (1.B,D) of a-46-year-old male with organized pneumonia in left upper lobe, Ktrans0.07, Kep0.31, Ve0.227, iAUC 8.438. Significant differences were observed in Ktrans and iAUC between malignancies and benign pulmonary lesions.Conclusion
Significant Ktrans, Kep, and iAUC differences were found between malignant and benign pulmonary lesions on DCE-MR scanning. DCE-MR is a viable and effective tool for the differentiation of benign and malignant pulmonary lesions.Acknowledgements
No acknowledgement found.References
1. Wahidi MM, Govert JA, Goudar RK, Gould MK, McCrory DC. Evidence for the treatment of patients with pulmonary nodules: when is it lung cancer?: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007; 132:94s-107s
2. Jeong YJ, Lee KS, Jeong SY, et al. Solitary pulmonary nodule: characterization with combined wash-in and washout features at dynamic multi-detector row CT. Radiology 2005; 237:675-683
3. Karaman A, Araz O, Durur-Subasi I, et al. Added value of DCE-MRI in the management of cystic-cavitary lung lesions. Respirology 2016; 21:739-745
4. Ohno Y, Koyama H, Takenaka D, et al. Dynamic MRI, dynamic multidetector-row computed tomography (MDCT), and coregistered 2-[fluorine-18]-fluoro-2-deoxy-D-glucose-positron emission tomography (FDG-PET)/CT: comparative study of capability for management of pulmonary nodules. Journal of magnetic resonance imaging : JMRI 2008; 27:1284-1295