2458
The Impact of Inspiration Levels on the Repeatability of Quantitative Pulmonary Perfusion DCE-MRI in Patients with Chronic Obstructive Pulmonary Disease and Cystic Fibrosis1Transl. Medicine + Clin. Pharmacology, Boehringer Ingelheim Pharma GmbH & Co. KG, Biberach an der Riss, Germany, 2Diagnostic and Interventional Radiology, University Hospital of Heidelberg, Heidelberg, Germany, 3Translational Lung Research Center Heidelberg (TLRC), German Lung Research Center (DZL), Heidelberg, Germany, 4Diagnostic and Interventional Radiology with Nuclear Medicine, Thoraxklinik at the University Hospital of Heidelberg, Heidelberg, Germany, 5Pulmonology and Respiratory Medicine, Thoraxklinik at the University Hospital of Heidelberg, Heidelberg, Germany, 6Pediatric Pulmonology & Allergy and Cystic Fibrosis Center, Pediatrics, University of Heidelberg, Heidelberg, Germany, 7Translational Pulmonology, University Hospital Heidelberg, Heidelberg, Germany, 8Diagnostic and Interventional Radiology, Hufeland Hospital, Bad Langensalza, Germany
Synopsis
The objective of this study was to investigate the 4-week repeatability of contrast-agent based pulmonary perfusion quantification in clinically stable patients with COPD and CF. Software including fully automated lung segmentation was used to determine pulmonary blood flow (PBF). While a good agreement of PBF was found in the majority of patients, high variabilities were found. Several influence factors were considered as explanations. Differences in SNR due to different inspiratory levels are likely to influence whether quantification in each voxel succeeds. Thus, it may be necessary to modify voxel-based quantification to compensate for differences in inspiratory levels and low SNR.
Introduction
The repeatability of software-based perfusion quantification of four-dimensional (4D) perfusion MRI was assessed in a small number of studies in healthy volunteers and patients with chronic obstructive pulmonary disease (COPD) [1]. Perfusion quantification is deemed an objective measure, but is hampered by the lack of standardised quantification algorithms and segmentation algorithms. Furthermore, the quantification of pulmonary perfusion is influenced by respiratory level [2], where scans acquired in inspiration lead to a lower repeatability compared to scans in expiration [1]. The objective of this study was to investigate the 4-week repeatability of pulmonary perfusion quantification in clinically stable patients with COPD and cystic fibrosis (CF) on maintenance therapy, and to assess factors influencing repeatability.Materials and Method
21 COPD and 15 CF patients completed two contrast-enhanced 4D perfusion MRI examinations each (MRI1 and MRI2) within 4 weeks with parallel clinical assessment and spirometry. All datasets were analysed using quantification software (PulmoMR, Fraunhofer Mevis, Germany) that performs fully automated lung segmentation and allows manual correction of lung contours where necessary. For 7 COPD and 4 CF patients no quantitative results could be generated due to insufficient contrast enhancement. Pulmonary blood flow (PBF) was calculated for the whole lung and for 12 automatically segmented lung regions of equal volume [3]. Differences in lung volume at MRI1 compared to MRI2 were used to assess differences in the inspiratory level. Statistical analyses were performed using R project for statistical computing (R 3.3.2 Foundation for Statistical Computing, Vienna, Austria).Results
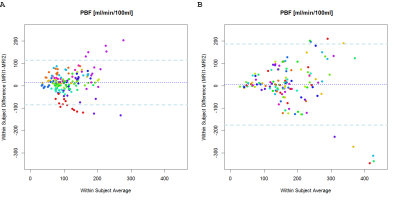
In Figure 1 the mean differences in PBF between the examinations at MRI1 and MRI2 are shown, with a good agreement of PBF in the majority of patients. The mean difference of PBF in COPD patients was 14.81 ± 49.7 ml/min/100ml and the mean difference of PBF in CF patients was 6.02 ± 90.58 ml/min/100ml.
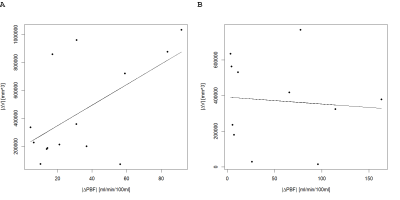
The relationship of the absolute differences in lung volume and PBF between MRI1 and MRI2 are shown in Figure 2. In COPD patients increasing differences in lung volumes correspond to increasing differences in PBF between MRI1 and MRI2 in absolute values, but no relationship can be seen in CF patients.
Discussion
This study investigated the repeatability of pulmonary perfusion quantification after 4 weeks. Disease-related changes between the examinations were excluded by spirometry and clinical assessments. Furthermore, technical confounders were reduced by using the same acquisition protocol and scanner. Although the medians of PBF were not significantly different between measurements, the observed standard deviations and limits of agreement indicate high inter-individual variation in the repeatability of the method. The variability in PBF might be caused by differences in the inspiratory level and the voxel-based quantification algorithm. It is known that a higher inspiratory level leads to lower blood volume per voxel resulting in decreased SNR. In voxels with extremely low SNR, the determination of PBF must fail altogether since the shape of the curve cannot be discerned due to noise. The SNR in the lungs of COPD patients is further reduced by disease, while in CF patients proton density may actually increase, both of which introduce different biases into the failure of local quantification. In COPD patients this could make PBF harder to use as disease monitoring marker, because the voxels with the lowest proton density and thus voxels with the worst SNR are also likely the most diseased ones. In addition, the segmentation algorithm requirement that all 12 regions have to be of equal size leads to changes in the regions’ localisation at different inspiratory levels. In particular, voxels of large vessels with high levels of PBF can be included into different regions, which modifies quantification results.Conclusions
Voxel-based quantification may be modified to compensate for differences in inspiratory levels and the low lung SNR. Reducing the variability of pulmonary perfusion quantification is a prerequisite for monitoring therapy response, or to differentiate between regions of reversible and irreversible disease.
Acknowledgements
No acknowledgement found.References
1. Ley-Zaporozhan, J., et al., Repeatability and reproducibility of quantitative whole-lung perfusion magnetic resonance imaging. J Thorac Imaging, 2011. 26(3): p. 230-9.
2. Fink, C., et al., Effect of inspiratory and expiratory breathhold on pulmonary perfusion: assessment by pulmonary perfusion magnetic resonance imaging. Invest Radiol, 2005. 40(2): p. 72-9.
3. Kohlmann, P., et al., Automatic lung segmentation method for MRI-based lung perfusion studies of patients with chronic obstructive pulmonary disease. Int J Comput Assist Radiol Surg, 2015. 10(4): p. 403-17.
Figures