2457
Implementation of the FLORET Ultrashort Echo-Time Sequence for Lung Imaging1Center for Pulmonary Imaging Research, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, United States, 2Phoenix Children’s Hospital, Phoenix, AZ, United States, 3Philips Healthcare, Gainesville, FL, United States, 4Barrow Neurological Institute, Phoenix, AZ, United States, 5Department of Radiology, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States
Synopsis
MRI of lungs is inherently challenging due to the short T2* and intrinsic motion from the respiratory and cardiac cycles. Ultrashort echo-time (UTE) sequences are often implemented for their shorter echo times and relative insensitivity to motion. Spiral UTE sequences have been touted recently as having greater k-space sampling efficiencies than radial UTE, but few are designed well for the shorter T2* of lung. In this study, FLORET (Fermat looped, orthogonally encoded trajectories), a recently-developed spiral 3D UTE sequence, was implemented in human lungs for the first time and outperformed traditional radial UTE for imaging of lung tissue.
Purpose:
Diagnostic-quality MRI of the lungs is challenging due to low lung parenchymal proton densities, intrinsic physiologic motion (respiratory/cardiac cycles), and inherently short T2* of the lung parenchyma (approximately 0.75 msec at 3T1). Ultrashort echo-time (UTE) MRI sequences overcome the loss of signal due to T2* by shortening the echo time to less than 100 µs in many cases. However, most radial UTE acquisitions require long scan times and oversample small k-values. Spiral trajectories to acquire k-space have been shown to be more robust to motion artifacts2 but typically require long T2* components to image well. Recently, FLORET (Fermat looped, orthogonally encoded trajectories), a spiral acquisition technique, was developed and exhibits high signal-to-noise and sampling efficiencies compared standard 3D UTE sequences.3,4 We demonstrate the ability of the FLORET sequence to obtain faster and higher-quality lung images in human subjects compared to traditional “fast” UTE techniques (here, stack-of-stars (SOS)).Methods:
FLORET is based on the Fermat spiral to avoid oversampling low k values, allowing for higher sampling efficiencies4. Each spiral is projected onto a single cone (between +45° and -45°). Two orthogonal sets of cones were acquired to fully measure k-space. The initial angle of the spiral out of k=0 was rotated via the golden angle. Additionally, rapid gradient spoiling (directly after the readout), was implemented.
The FLORET sequence calculates ideal spiral parameters to maximize sampling efficiency for a given systems gradient amplitude and slew rate (31 mT/m and 74 mT/m/s used here, respectively on Philips 3.0 T, R5.1.7). The chest of a healthy adult male subject (age 33) were imaged using a Philips 32 channel anterior/posterior chest coil during free-breathing. The scan parameters were: 0.093 ms echo time (TE), 5° flip angle, 3.92 ms repetition time (TR), 1.489 mm isotropic voxels, 268 isotropic matrix, 43,112 total acquisitions, and a 100% sampled k-space. The full acquisition of FLORET took just under 3 minutes.
Radial stack-of-stars was acquired on the same subjects with the same parameters, coil, and magnet, though the minimum TE was very slightly greater, at 0.122 ms. SOS allows for one non-isotropic dimension, allowing for fewer slices (160) in the foot to head dimension, thus saving time. However, the full image required 85,760 FID acquisitions and 7 minutes for the full acquisition. All acquisitions were gridded and reconstructed similarly using Graphical Programming Interface (GPI).5 The GPI reconstruction implemented a sampling-density correction to grid the acquisitions into a Cartesian matrix. Following 3D FFT, images were corrected for off-resonance effects and B1 inhomogeneity.
Results:
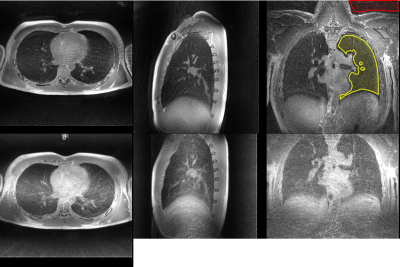
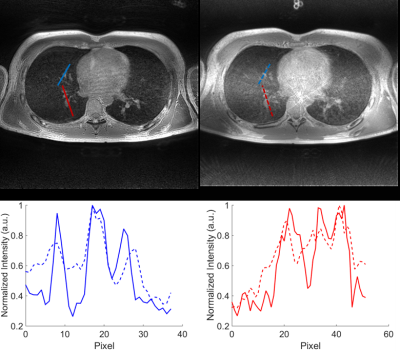
Both acquisition techniques result in diagnostic-quality lung images, with parenchyma signal visible, as seen in Figure 1, despite the 2.5x difference in acquisition length. Comparing the images, FLORET outperforms SOS in some image quality metrics. The signal to noise ratio (SNR) of the lung parenchyma was 4.3 for FLORET and 2.9 for SOS UTE, and the parenchyma/muscle ratios were 0.34 for FLORET and 0.42 for SOS. The vasculature was visualized with greater contrast using FLORET, as illustrated in Figure 2. SOS UTE exhibited stronger non-Cartesian artifacts compared to FLORET, as seen near the heart in the axial views of Figures 1 and 2. However, the proprietary image reconstruction removes the artifacts and improves SNR. Both methods appear to perform similarly with respect to motion artifacts during these free breathing images, though FLORET appears to have more noticeable off-resonance affects (most notable near the spine in these images), which may be overcome by advanced focusing techniques. Importantly, the SNR and vascular contrast per rf excitation was higher in FLORET than in SOS, providing a time and/or spatial resolution advantage.Discussion:
By using GPI-reconstruction, direct comparisons of image quality for the sequences can be performed. FLORET was near-equivalent to SOS UTE even with a much smaller number of k-space trajectories. Non-Cartesian artifacts are much less noticeable with FLORET but the longer FID acquisition time makes the off-resonance effects more noticeable. Importantly, the ~2.5 times faster image acquisition (at identical spatial resolutions) is ideal for less-compliant or pediatric patients.Conclusion:
The FLORET sequence allows for faster acquisition of high diagnostic-quality lung images and its short T2* components without sacrificing signal to noise or quantification of abnormalities. This sequence has potential for clinical and research translation in pulmonary MRI, particularly in pediatric or less-compliant adult patients with the shortened scan time. The ability to quantify lung parenchyma accurately with a short scan time can facilitate phenotyping in both emphysematous (e.g. COPD, BPD) and fibrotic (e.g. IPF, NSIP) pulmonary conditions.Acknowledgements
The authors thank the following sources for research funding and support: NIH R01 HL131012 and NIH R44 HL123299. Additionally, the authors would like to thank Ashley G. Anderson III PhD (Philips Healthcare) for the ability to implement their off resonance focusing code.References
1. Roach DJ, Crémillieux Y, Serai SD, et al. Morphological and quantitative evaluation of emphysema in chronic obstructive pulmonary disease patients: A comparative study of MRI with CT. J Magn Reson Imaging. 2016;44(6):1656-1663. doi:10.1002/jmri.25309.
2. Salerno M, Altes TA, Brookeman JR, Lange EE De, Mugler III JP. Dynamic Spiral MRI of Pulmonary Gas Flow Using Hyperpolarized 3He : Preliminary Studies in Healthy and Diseased Lungs. Magn Reson Med. 2001;46:667-677.
3. Pipe JG, Zwart NR, Aboussouan EA, Robison RK, Devaraj A, Johnson KO. A new design and rationale for 3D orthogonally oversampled k-space trajectories. Magn Reson Med. 2011;66(5):1303-1311. doi:10.1002/mrm.22918.
4. Robison RK, Anderson AG, Pipe JG. Three-dimensional ultrashort echo-time imaging using a FLORET trajectory. Magn Reson Med. 2017;78(3):1038-1049. doi:10.1002/mrm.26500.
5. Zwart NR, Pipe JG. Graphical programming interface: A development environment for MRI methods. Magn Reson Med. 2015;74(5):1449-1460. doi:10.1002/mrm.25528.
Figures