2434
Observing Pulmonary Gas-Transport Dynamics Using Rapid 1D Hyperpolarized Xenon-129 Dissolved-Phase MeasurementsKai Ruppert1, Hooman Hamedani1, Faraz Amzajerdian1, Luis Loza1, Yi Xin1, Ian F. Duncan1, Harilla Profka1, Sarmad Siddiqui1, Mehrdad Pourfathi1, Stephen Kadlecek1, and Rahim R. Rizi1
1Radiology, University of Pennsylvania, Philadelphia, PA, United States
Synopsis
Monitoring the dissolved xenon-129 signal in a central downstream location such as the left ventricle of the heart provides a convenient measure of the lung’s gas transport dynamics, and thereby of total lung function. To demonstrate the feasibility of this approach, we combined a rapid simultaneous gas-phase / dissolved-phase 1D-projection acquisition with regional gas-phase saturation to monitor the gas-transport dynamics of the lung as signal variations in the heart of a rat model of radiation-induced lung injury. Our measurements indicate that this method can identify the reductions in regional lung function associated with partial lung irradiation.
Purpose
Hyperpolarized xenon-129 (HXe) readily dissolves in the blood upon inspiration, and is subsequently transported throughout the body via pulmonary circulation. Monitoring the dissolved xenon-129 signal in a central downstream location such as the left ventricle of the heart therefore provides a convenient measure of the lung’s gas transport dynamics, and thereby of total lung function. To demonstrate the feasibility of this approach, we combined a rapid simultaneous gas-phase (GP) / dissolved-phase (DP) 1D-projection acquisition with regional gas-phase saturation to monitor the gas-transport dynamics of the lung as signal variations in the heart of a rat model of radiation-induced lung injury.Methods
The lungs of 3 Fisher rats (approx. 300 g) were primed with an intratracheal instillation of lipopolysaccaride (10 mg/mL), followed by right hemi-thorax radiation (25Gy) after 24 hours. Imaging studies were conducted one month post-radiation, when early fibrosis can typically be detected. One healthy Fisher rat was imaged as a control. The sedated animals were ventilated with room air until imaging began, at which point the gas mix was switched to 20% oxygen and 80% HXe for four wash-in breaths (6 ml/kg tidal volume); the acquisition was started on the fifth breath. Separated by another wash-in breath, each acquisition was repeated three more times and the measurements averaged. All studies were approved by the Institutional Animal Care and Use Committee. MR imaging was conducted using a 1D-projection gradient-echo sequence that employed a non-selective 900-μs Gaussian RF excitation pulse centered 3,530 Hz downfield from the gas-phase resonance. Taking advantage of the large frequency difference between the two phases combined with a sufficiently small acquisition bandwidth, signal from HXe in both the pulmonary air spaces and dissolved in the lung tissue was imaged simultaneously, side-by-side1. The following sequence parameters were used: readout direction left to right; 80 samples per readout repeated 100 times; TR/TE 50/1.7 ms; flip angle 10°; FOV 220 mm; receiver bandwidth 110 Hz/pixel. Three 1D projection measurements were performed in each animal: 1) without regional gas-phase saturation; 2) after saturation of the gas phase in the left lung; 3) after saturation of the gas phase in the right lung. All MR studies were performed at 1.5T (Avanto; Siemens), using a custom xenon-129 transmit/receive birdcage coil (Stark Contrast, Erlangen, Germany). Enriched xenon gas (87% xenon-129) was polarized using a prototype commercial system (XeBox-E10, Xemed LLC, Durham, NH).Results and Discussion
Figure 1 illustrates the principles underlying the conducted dynamic 1D acquisitions. Figure 1A shows a conventional 2D-coronal simultaneous GP/TP projection acquisition in a healthy rat using a pulse sequence similar to that proposed by Mugler et al1; Fig. 1B depicts an equivalent measurement in the same animal without phase encoding along the apex to base direction. One of the most prominent features of both measurements is the strong dissolved-phase signal from the left ventricle of the heart, corresponding to the tall peak at image column 22 in the 1D projection. The evolution of the signal in Fig. 1B over the course of a 5-s breath hold is demonstrated in Fig. 1C. Of particular interest for our study is the image column dominated by the signal from the heart, which builds up to a peak during the early phase of the breath hold and then slowly decays. Figure 2 shows the signal dynamics of the same image column after saturation of the GP magnetization of either the left or right lung 750 ms into the breath hold. The heart signal drops by about 1/3 after GP saturation of either lung in the healthy animal. In the irradiated rat, on the other hand, saturation of the GP in the left lung is associated with a rapid, large signal drop, but a much smaller, more gradual decrease following GP saturation in the irradiated right lung. This finding indicates that right lung function has been severely compromised, with the left lung providing the bulk of the remaining functionality. Qualitatively similar results were found in all irradiated animals.Conclusion
We demonstrated the implementation of a rapid 1D GP-DP acquisition technique combined with regional GP saturation to monitor the gas transport dynamics of the lung. Our measurements indicate that this method can identify the reductions in regional lung function associated with partial lung irradiation. Although the absolute HXe signal from the heart is obscured by the presence of overlapping parenchyma signals in a 1D projection, the relative changes in signal as a function of time permit the isolation of the heart signal.Acknowledgements
Supported by NIH grants R01 EB015767 and R01 HL129805.References
[1] Mugler et al. Simultaneous magnetic resonance imaging of ventilation distribution and gas uptake in the human lung using hyperpolarized xenon-129. Proc Natl Acad Sci USA 2010;107(50):21707-21712.Figures
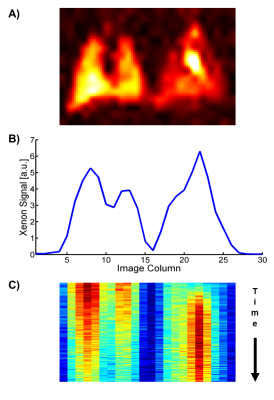
Figure 1. Characterization of the xenon-129 uptake dynamics in a healthy rat lung. A) 2D coronal projection image of the simultaneously acquired xenon GP (left) and DP distribution. B) 1D projection data of an acquisition identical to (A) but without phase encoding in the apex-to-base direction. C) Color-coded time evolution of the xenon-129 signal in the 1D projection data with each horizontal line in the image representing a time increment of 50 ms.
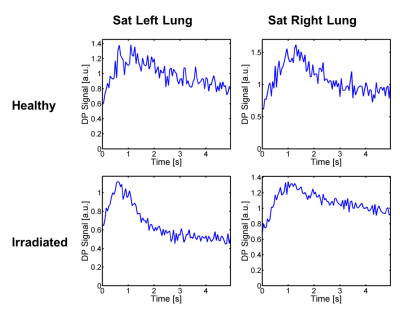
Figure 2. Temporal dynamics of the data point in the 1D projection acquisition that contains the bulk of the xenon-129 signal from the left ventricle following saturation of the gas-phase magnetization in either the left or the right lung. Identical measurements were conducted in a healthy control rat (upper panels) and a rat with an irradiated right lung (lower panels). In the healthy animal both lungs showed qualitatively similar gas transport characteristics. In the irradiated animal the injured right lung exhibited considerably reduced gas transport, which seemed to be at least partially compensated for by an increased gas transport in the left lung.