Synopsis
Molecular
subtype classification of breast tumor is of paramount importance in
determining aggressiveness and prognosis. The ability to use diffusion weighted
imaging (DWI) for the prediction of molecular subtypes may improve management
in breast cancer. In this study, two radiologists retrospectively evaluated
different metrics on apparent diffusion coefficient maps of 107 patients with
invasive breast cancer. ER and PR positive lesions had lower ADC values while
HER2 positive and high-proliferating had higher values. Luminal cancers had
lower ADC values than other subtypes, thus DWI may be used to predict
tumor subtype in breast cancer.
Introduction
Magnetic
resonance imaging (MRI) is an essential tool for the diagnosis and staging of
breast cancer (1,
2). To improve limitations on specificity, diffusion weighted
imaging (DWI) has emerged as a robust MRI parameter. DWI measures the random
motion of water molecules, which can be quantified by the apparent diffusion
coefficient (ADC) (3-7).
Immunohistochemical
(IHC) receptor status, i.e presence of estrogen receptor (ER), progesterone receptor
(PR), human epidermal
growth factor receptor 2 (HER2) and proliferation rate (Ki-67) are major prognostic
factors and are predictive of response to neoadjuvant treatment. Based on
IHC-surrogates, molecular subtypes can be defined (8,
9). However, to date the receptor status and
proliferation rate has to be obtained by tissue sampling and up to 20% of
patients show disagreement between biopsy and surgical specimen (10-12).
ADC
has been investigated for predicting IHC status but results are divergent (12-21). Therefore, the aim of the study was to evaluate whether
different ADC metrics can be used for prediction of IHC status and
molecular subtype in breast cancer.Material and Methods
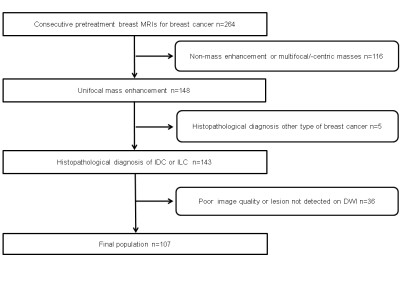
The
Institution Review Board approved this retrospective study, which selected patients
who underwent MRI of the breast with DWI between 12/2010 and 02/2014 and
fulfilled the following inclusion criteria: histopathologically verified
invasive breast cancer, 18 years or older; not pregnant or breastfeeding; no
previous treatment; and no contraindications for MRI or contrast agents. The exclusion
criteria were 1) non-mass enhancement or multiple masses, 2) Other types of
cancer than invasive ductal carcinoma (IDC) or invasive lobular carcinoma (ILC),
3) poor image quality or lesion not detected on DWI. Overall, 107 patients were
included in the study (Figure 1).
MRI studies were independently
evaluated by two
board-certified radiologists with 6 (reader 1) and 12 years (reader 2) of
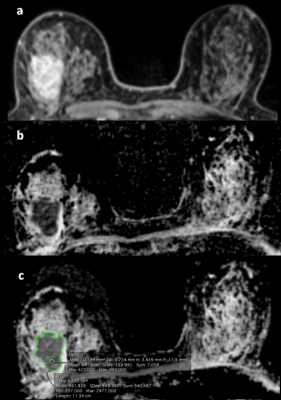
experience in breast MR imaging. The tumors were identified on high-b-value (850 s/mm2) using DCE-MR images as anatomical
guidance. The radiologists chose the
slice with the greatest representative portion of the tumor and drew one 2-dimensional
region of interest (ROI) on the whole tumor (WTu) on ADC maps, and another
2-dimensional ROI on the darkest part (Dp) of the tumor (Figure 2). The
minimum, mean and maximum ADC values of both the WTu and Dp were compared with histopathology,
grade, ER, PR, HER 2 and Ki-67 status and molecular subtype.
Results
ER,
PR and HER2 status was available in all 107 patients, and Ki-67 in 87. ER, PR
positive tumors had lower ADC values, while HER2 positive and KI-67
high-proliferating lesions had higher values. Differentiation among molecular
subtypes were statistically significant for both readers. Luminal tumors had
lower ADC values while HER2-enriched tumors had the highest. Differentiation
between luminal tumors and other subtypes were possible for Dp mean, Dp
minimum, WTu mean, WTu minimum and WTu maximum measurements for both readers
(p<0.0001). The areas under the curve were higher for measurements of the
WTu mean (0.685 for both readers) and WTu maximum (0.606 for reader 1 and 0.627
for reader 2). Comparing luminal A tumors, that had the lowest values, with all
other subtypes combined, we found that all measurements were statistically
significant for both readers (p<0.0001). The areas under the curve were also
higher for measurements of the WTu mean (0.647 for reader 1 and 0.685 for
reader 2) and WTu maximum (0.698 for
reader 1 and 0.659 for reader 2).Discussion
The
ADC values were lower in ER and PR positive and higher in HER2 positive and
high-proliferating tumors. ADC values were significantly different among
molecular subtypes and could differentiate luminal tumors from all other
subtypes. The diagnosis of luminal A lesions is of paramount clinical
importance since they might benefit less from neo- and adjuvant
chemotherapy.
The
increased cellularity seen in most breast cancers causes a restriction in the
movement of water particles and reduces the ADC values (13). Therefore, high-proliferating tumors are expected
to have lower ADC than low-proliferating tumors. On the other hand, a greater
amount of extracellular fluid due to an increased neovacularity with greater
permeability in a tumor can increase ADC values, a phenomenon which has been
observed in HER2 positive lesions (16,
21). In contrast, ER positive tumors, tend to have less
neovascularity and therefore lower ADC values, as observed in our study (13,
16). Conclusion
In
conclusion, ADC measurements have the potential to non-invasively determine IHC
status and differentiate molecular subtypes.Acknowledgements
No acknowledgement found.References
1. Mann RM, Balleyguier C, Baltzer PA,
Bick U, Colin C, Cornford E, et al. Breast MRI: EUSOBI recommendations for
women's information. European radiology. 2015;25(12):3669-78.
2. Sardanelli
F, Boetes C, Borisch B, Decker T, Federico M, Gilbert FJ, et al. Magnetic
resonance imaging of the breast: recommendations from the EUSOMA working group.
European journal of cancer. 2010;46(8):1296-316.
3. Partridge
SC, DeMartini WB, Kurland BF, Eby PR, White SW, Lehman CD. Quantitative
diffusion-weighted imaging as an adjunct to conventional breast MRI for
improved positive predictive value. AJR American journal of roentgenology.
2009;193(6):1716-22.
4. Pinker
K, Helbich TH, Morris EA. The potential of multiparametric MRI of the breast.
The British journal of radiology. 2017;90(1069):20160715.
5. Tan
SL, Rahmat K, Rozalli FI, Mohd-Shah MN, Aziz YF, Yip CH, et al. Differentiation
between benign and malignant breast lesions using quantitative
diffusion-weighted sequence on 3 T MRI. Clinical radiology. 2014;69(1):63-71.
6. Cheeney
S, Rahbar H, Dontchos BN, Javid SH, Rendi MH, Partridge SC. Apparent diffusion
coefficient values may help predict which MRI-detected high-risk breast lesions
will upgrade at surgical excision. Journal of magnetic resonance imaging :
JMRI. 2017.
7. Bickel
H, Pinker K, Polanec S, Magometschnigg H, Wengert G, Spick C, et al.
Diffusion-weighted imaging of breast lesions: Region-of-interest placement and
different ADC parameters influence apparent diffusion coefficient values.
European radiology. 2017;27(5):1883-92.
8. Cipolla
V, Santucci D, Guerrieri D, Drudi FM, Meggiorini ML, de Felice C. Correlation
between 3T apparent diffusion coefficient values and grading of invasive breast
carcinoma. European journal of radiology. 2014;83(12):2144-50.
9. Baba
S, Isoda T, Maruoka Y, Kitamura Y, Sasaki M, Yoshida T, et al. Diagnostic and
prognostic value of pretreatment SUV in 18F-FDG/PET in breast cancer:
comparison with apparent diffusion coefficient from diffusion-weighted MR
imaging. J Nucl Med. 2014;55(5):736-42.
10. Mann
GB, Fahey VD, Feleppa F, Buchanan MR. Reliance on hormone receptor assays of
surgical specimens may compromise outcome in patients with breast cancer. J
Clin Oncol. 2005;23(22):5148-54.
11. Burge
CN, Chang HR, Apple SK. Do the histologic features and results of breast cancer
biomarker studies differ between core biopsy and surgical excision specimens?
Breast. 2006;15(2):167-72.
12. Martincich
L, Deantoni V, Bertotto I, Redana S, Kubatzki F, Sarotto I, et al. Correlations
between diffusion-weighted imaging and breast cancer biomarkers. Eur Radiol.
2012;22(7):1519-28.
13. Choi
SY, Chang YW, Park HJ, Kim HJ, Hong SS, Seo DY. Correlation of the apparent
diffusion coefficiency values on diffusion-weighted imaging with prognostic
factors for breast cancer. The British journal of radiology.
2012;85(1016):e474-9.
14. Guvenc
I, Akay S, Ince S, Yildiz R, Kilbas Z, Oysul FG, et al. Apparent diffusion
coefficient value in invasive ductal carcinoma at 3.0 Tesla: is it correlated
with prognostic factors? The British journal of radiology.
2016;89(1060):20150614.
15. Jeh
SK, Kim SH, Kim HS, Kang BJ, Jeong SH, Yim HW, et al. Correlation of the
apparent diffusion coefficient value and dynamic magnetic resonance imaging
findings with prognostic factors in invasive ductal carcinoma. Journal of
magnetic resonance imaging : JMRI. 2011;33(1):102-9.
16. Karan
B, Pourbagher A, Torun N. Diffusion-weighted imaging and (18)
F-fluorodeoxyglucose positron emission tomography/computed tomography in breast
cancer: Correlation of the apparent diffusion coefficient and maximum
standardized uptake values with prognostic factors. Journal of magnetic
resonance imaging : JMRI. 2016;43(6):1434-44.
17. Kim
SH, Cha ES, Kim HS, Kang BJ, Choi JJ, Jung JH, et al. Diffusion-weighted
imaging of breast cancer: correlation of the apparent diffusion coefficient
value with prognostic factors. Journal of magnetic resonance imaging : JMRI.
2009;30(3):615-20.
18. Kitajima
K, Yamano T, Fukushima K, Miyoshi Y, Hirota S, Kawanaka Y, et al. Correlation of
the SUVmax of FDG-PET and ADC values of diffusion-weighted MR imaging with
pathologic prognostic factors in breast carcinoma. European journal of
radiology. 2016;85(5):943-9.
19. Lee
HS, Kim SH, Kang BJ, Baek JE, Song BJ. Perfusion Parameters in Dynamic Contrast-enhanced
MRI and Apparent Diffusion Coefficient Value in Diffusion-weighted MRI::
Association with Prognostic Factors in Breast Cancer. Academic radiology.
2016;23(4):446-56.
20. Nakajo
M, Kajiya Y, Kaneko T, Kaneko Y, Takasaki T, Tani A, et al. FDG PET/CT and
diffusion-weighted imaging for breast cancer: prognostic value of maximum
standardized uptake values and apparent diffusion coefficient values of the
primary lesion. Eur J Nucl Med Mol Imaging. 2010;37(11):2011-20.
21. Park
SH, Choi HY, Hahn SY. Correlations between apparent diffusion coefficient
values of invasive ductal carcinoma and pathologic factors on
diffusion-weighted MRI at 3.0 Tesla. Journal of magnetic resonance imaging :
JMRI. 2015;41(1):175-82.