2427
Ultra-high field Dynamic Contrast Enhanced Magnetic Resonance Imaging of the Breast with pharmacokinetic (PK) modeling: Value for the Differentiation of Benign and Malignant Breast Tumors and Molecular Breast Cancer SubtypesRosa Elena Ochoa Albiztegui1, Joao Vicente Machado Horvat1, Sunitha Thakur1, Blanca Bernard-Davila1, Siegfried Trattnig2, Thomas Helbich2, Elizabeth Morris1, and Katja Pinker-Domenig1
1Radiology, Memorial Sloan Kettering Cancer Center, New York, NY, United States, 2Biomedical Imaging and Image-guided Therapy, Medical University Vienna, Vienna, Austria
Synopsis
To investigate ultra-high field DCE-MRI of the breast at 7T with pharmacokinetic modeling for differentiation of benign and malignant breast tumors and molecular breast cancer subtypes. 37 patients with 43 breast lesion were included and underwent a 7T DCE-MRI of the breast. Quantitative pharmacokinetic imaging biomarkers ktrans and kep aid in the differentiation of benign and malignant breast tumors. Selection of ROI- using a whole tumor and a 10mm2 ROI- does not influence diagnostic accuracy. Quantitative pharmacokinetic imaging biomarkers ktrans and kep are not able to differentiate molecular breast cancer subtypes.
Background
Dynamic contrast-enhanced (DCE) MRI is the most sensitive imaging technique for breast cancer diagnosis. DCE-MR can depict and characterize this abnormal vasculature and permeability as a tumor-specific feature through the assessment of breast kinetic enhancement features. High temporal resolution MRI techniques enable a quantitative kinetic enhancement analysis through pharmacokinetic modelling. Pharmacokinetic models quantify the contrast agent exchange between the intravascular and the interstitial space, providing measures of tumor blood flow, the microvasculature, and capillary permeability as thus may provide valuable information for differentiation of benign and malignant breast tumors as well as different molecular breast cancer subtypes.Purpose
To assess if ultra-high field dynamic contrast enhanced magnetic resonance imaging of the breast at 7T with pharmacokinetic modeling (PK) can aid differentiation of benign and malignant breast tumors and molecular breast cancer subtypes.Materials and Methods
In this this IRB-approved study 37 patients with a suspicious imaging finding on mammography or ultrasound (BI-RADS 4,5) were included and underwent a 7T DCE-MRI of the breast using a high temporal and spatial resolution sequence (temporal resolution of 14 s, spatial resolution of 0.7 mm3 voxel size). Quantitative PK imaging biomarkers Ktrans and kep were assessed using the DCE-Tool OsiriX plugin http://kyungs.bol.ucla.edu/software/DCE_tool/DCE_tool.html). Two independent readers manually draw a whole tumor 2D-ROI (wtROI) and a 10mm2 standardized 2D-ROI (standROI) in the MRI slice with maximum lesion diameter and most enhancing part of the lesion. Histopathology was used as the standard of reference. In case of a malignant breast tumor molecular breast cancer subtypes were derived via IHC surrogates. Tumors were classified as luminal A if either ER or PR was positive and HER2 was negative, Luminal B if either ER or PR was positive and HER2 positive, HER2-enriched if ER and PR were negative and HER2 positive and basal-like if ER, PR and HER2 were negative. Pearson, Spearman’s and Kendall’s correlations coefficient were used to assess correlation between two readers. As correlation coefficient does not assess variability, that is, linear error between the 2 readers, Bland–Altman test was used to evaluate the inter-reader agreement. ANOVA and Kruskal-Wallis h test were applied to identify association between Molecular Subtypes and pharmacokinetics (ktrans and kep). Independent samples t-test and Wilcoxon rank-sum (Mann-Whitney) test were used with ktrans and kep to differentiate between malignant and benign lesions.Results
There were 43 lesions, 17 benign and 26 malignant (table 1). PK modeling using either a wtROI and standROI could not differentiate between molecular breast cancer subtypes: ktrans (p=0.854 and p=0.366) and kep (p=0.733 and p= 0.585). KTrans for malignant and benign lesions were significantly different in the wtROI (p=0.0070) and stand ROI (p=0.0023). Kep for malignant and benign lesions were also significantly different in the wtROI (p=0.0050) and stand ROI (p=0.0149). The four measurements, whole tumor region of interest KTrans (wtROI-KTrans), 10mm2 standardized 2D-ROI KTrans (sROI-Ktrans), whole tumor region of interest kep (wtROI-kep) and 10mm2 standardized 2D-ROI kep (sROI-kep) had excellent inter-reader agreement and correlation between the 2 readers was 91%, 88%, 84%, 76%, respectively (p=<0.01). Bland–Altman test confirmed a good inter-reader agreement.Conclusion
Quantitative pharmacokinetic imaging biomarkers ktrans and kep aid in the differentiation of benign and malignant breast cancer diagnosis. Selection of ROI- using a whole tumor and a standardized 10mm2 ROI- does not influence diagnostic accuracy. Quantitative pharmacokinetic imaging biomarkers ktrans and kep are not able to differentiate molecular breast cancer subtypes.Acknowledgements
No acknowledgement found.References
- D’Orsi CJ, Sickles EA, Mendelson EB, Morris EA, al. e. “ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System.” 5th ed: Reston, VA, American College of Radiology, 2013.
- Yim et al., “Analysis of Kinetic Curve and Model-Based Perfusion Parameters on Dynamic Contrast Enhanced MRI in Breast Cancer Patients: Correlations with Dominant Stroma Type.”
- Braman et al., “Intratumoral and Peritumoral Radiomics for the Pretreatment Prediction of Pathological Complete Response to Neoadjuvant Chemotherapy Based on Breast DCE-MRI.”
- Li et al., “Application of Whole-Lesion Histogram Analysis of Pharmacokinetic Parameters in Dynamic Contrast-Enhanced MRI of Breast Lesions with the CAIPIRINHA-Dixon-TWIST-VIBE Technique.”
- Pinker, Helbich, and Morris, “The Potential of Multiparametric MRI of the Breast.”
- Shafi et al.,
“Omics Approaches in Breast Cancer.”
Figures
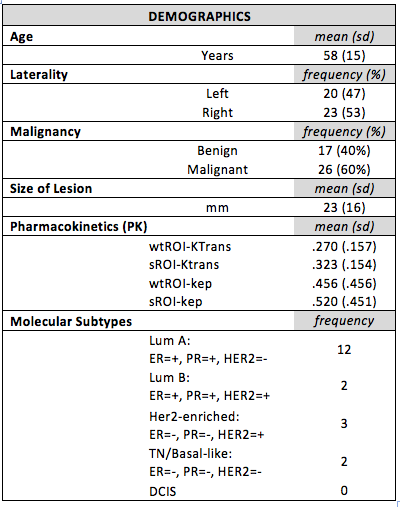
Demographics
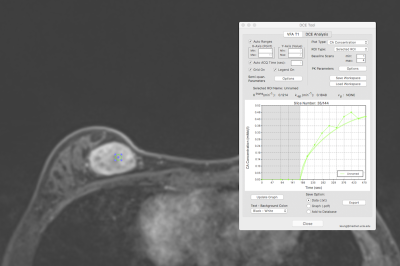
Benign: Pharmacokinetics of a benign lesion, fibroadenoma. DCE
Tool where KTrans(min-1): 0.1214 and kep(min-1):
0.1848. 10mm2 ROI in slice with maximum lesion diameter and most
enhancing part of the lesion.
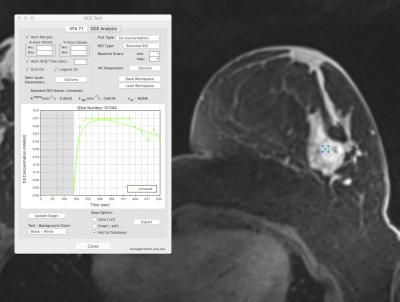
Malignant: Pharmacokinetics of a malignant lesion. DCE Tool
where KTrans(min-1): 0.3533 and kep(min-1):
0.8478. 10mm2 ROI in slice with maximum lesion diameter and most
enhancing part of the lesion.