2390
Investigating Local and Global Connectivity to Inform Seizure Generation in Epilepsy: a Feasibility Study1Medical Physics and Biomedical Engineering, University College London, London, United Kingdom, 2University of Verona, Verona, Italy, 3UCL Institute of Neurology, London, United Kingdom, 4King's College London, London, United Kingdom, 5UCLH Institute of Nuclear Medicine, London, United Kingdom, 6Great Ormond Street Hospital, London, United Kingdom
Synopsis
In this work, we combined local and global functional connectivity to provide a more complete picture of the epileptogenic brain in temporal lobe epilepsy (TLE). Local functional connectivity was assessed by computing regional homogeneity (ReHo) maps which were compared between left (n=9) or right (n=10) TLE patients and controls (n=20). Areas of increased ReHo were used in a seed-to-voxel analysis to investigate global functional connectivity changes. We report a different pattern of alteration between left and right TLE patients. Left TLE patients showed a more profound bilateral increased connectivity which might highlight compensatory mechanisms.
Introduction
The hyper-synchronicity of epileptic tissue during the interictal state is related to the subsequent seizure generation1. This local coherence of neural activity has been observed in the presumed epileptogenic network, however its relation to long range connectivity remains unknown1. Functional magnetic resonance (fMRI) based on the blood-oxygen-level dependent contrast is a non-invasive method which can capture spontaneous neural synchronisation at rest: functional connectivity (FC)1. This can be analysed at the local level, by exploiting measures of local signal coherence, or at the global level, using methodologies such as seed-based analysis which consider whole brain connectivity2.
In this study, we aimed at using areas of increased local synchrony to drive global FC analysis. This approach allows improved sensitivity of global FC analysis and decreased uncertainty in the seed definition2. This is the first study where this methodology has been applied to temporal lobe epilepsy (TLE), with patients divided accordingly to focus lateralisation, thereby providing a complete picture of the epileptogenic brain.
Methods
Nineteen TLE patients (7 females, 9 left-sided, 41±14y) and twenty matched healthy controls (9 females, 38±10y) were enrolled in this study. Imaging was carried out on a 3T Biograph Siemens PET/MR scanner. Resting-state fMRI data were acquired, with subjects’ eyes closed, using an echo-planar imaging sequence with: TR/TE=2020/30ms, 36 slices, voxel size=3x3x4mm3, 260 volumes. High resolution 3D T1-weighted MPRAGE anatomical images were also acquired.
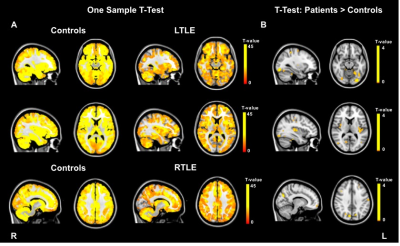
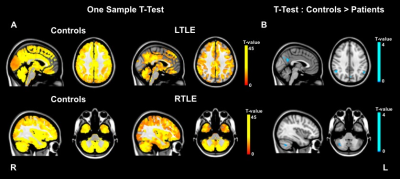
Local FC was assessed using regional homogeneity (ReHo), a measure of coherence of a given voxel time series with its neighbours’ time series3. Image pre-processing and ReHo computation were carried out using the toolbox DPARSFA (http://www.rfmri.org/DPARSF) based on SPM12 (http://www.fil.ion.ucl.ac.uk/spm/software/spm12/) with default pipeline. For each subject, ReHo maps were computed by automatically calculating the Kendall coefficient of concordance (KCC) of the time series of a given voxel with those of its 26 nearest neighbours in all directions3. KCC ranges between 0 (zero coherence) and 1 (perfect coherence). These ReHo maps were smoothed with a 6mm FWHM kernel, intensity normalised using the average cerebellum value for each subject and masked using a grey matter mask. A one-sample t-test was run to compute T-maps for controls, left TLE (LTLE) and right TLE (RTLE) (p-value<0.001, FWE-corrected and cluster extent k=10). A two-sample t-test was then run to detect regions of altered ReHo between patients (LTLE and RTLE, separately) and controls (patients>controls and controls>patients, p-value<0.01, uncorrected and cluster extent k=10).
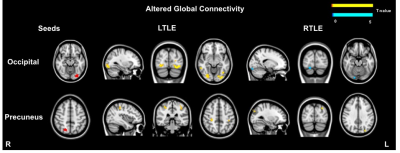
Global FC was assessed through seed-to-voxel analysis carried out in MATLAB using the toolbox CONNv.16 (https://www.nitrc.org/projects/conn), based on SPM12. The pre-processed data were smoothed (6mm FWHM) and used for seed-to-voxel analysis. The seeds were defined as the most significant clusters showing increased ReHo values (patients>controls). Single-subject FC maps were estimated by computing the correlation between the average signal in each seed and all the other brain voxels. These maps were compared between patients (LTLE and RTLE, separately) and controls using a two-sample t-test (voxel-wise threshold p-value<0.001, uncorrected and cluster-level threshold p-value<0.05, uncorrected).
Results
Increased ReHo (patients>controls) was found in the left occipital and insula in LTLE patients, while the right precuneus was detected in the RTLE group (Figure 1).
Decreased ReHo (controls>patients) was found in areas of the default mode network (DMN) in LTLE patients and in the right cerebellum in the RTLE group (Figure 2).
The regions of increased ReHo were used in a seed-to-voxel analysis to assess global connectivity changes. Figure 3 shows the areas of increased and decreased connectivity found in LTLE and RTLE for each seed. No significant altered global connectivity was found in the case of the insula. Of note, extensive increased connectivity was found between the left occipital lobe and other posterior regions, bilaterally in the LTLE group.
Discussion and Conclusions
Changes of ReHo were assessed for the first time in LTLE and RTLE, separately. Increased ReHo was found ipsilaterally in areas related to the epileptogenic network: occipital4, insula5 and precuneus5 (part of DMN). Decreased ReHo was reported in areas possibly disrupted as a consequence of the disease1. Overall, increased connectivity was found between the areas of increased ReHo and regions known to be disrupted and hyperconnected in TLE (visual and motor systems)4. The increased connectivity in LTLE between the left occipital area and the rest of the posterior lobe (bilateral) highlights a more profound interhemispheric alteration than RTLE, possibly related to reorganisation6. This is a pilot study where, in agreement with previous works, we report a different pattern of altered connectivity in LTLE when compared to RTLE, thereby confirming the different pathophysiology of these epilepsy types6.Acknowledgements
This work is supported by the EPSRC-funded UCL Centre for Doctoral Training in Medical Imaging (EP/L016478/1) and the Department of Health’s NIHR-funded Biomedical Research Centre at UCLH and GOSH.References
1. Zeng, H., Pizarro, R., Nair, V. A., La, C. & Prabhakaran, V. Altered regional homogeneity in patients with mesial temporal lobe epilepsy and hippocampal sclerosis. Epilepsia 54, 1–9 (2013).
2. Yan, F.-X. et al. Resting-State Functional Magnetic Resonance Imaging Analysis with Seed Definition Constrained by Regional Homogeneity. Brain Connect. 3, 438–449 (2013).
3. Zang, Y., Jiang, T., Lu, Y., He, Y. & Tian, L. Regional homogeneity approach to fMRI data analysis. Neuroimage 22, 394–400 (2004).
4. Liu, H. H. et al. Interhemispheric functional and structural alterations and their relationships with alertness in unilateral temporal lobe epilepsy. Eur. Rev. Med. Pharmacol. Sci. 20, 1526–1536 (2016).
5. Morgan, V. L., Abou-Khalil, B. & Rogers, B. P. Evolution of Functional Connectivity of Brain Networks and Their Dynamic Interaction in Temporal Lobe Epilepsy. Brain Connect. 5, 35–44 (2015).
6. Chiang, S., Stern, J. M., Engel Jr, J., Levin, H. S. & Haneef, Z. Differences in graph theory functional connectivity in left and right temporal lobe epilepsy. Epilepsy Res. 10, 1–20 (2016).
Figures