2389
Why elder adults have a higher fall risk in dual-task daily life: A Preliminary fMRI Study1Academy for Advanced Interdisciplinary Studies, Peking University, Beijing, China, 2Neuroscience and Intelligent Media Institute, Communication University of China, Beijing, China, 3Department of Radiology, Peking University First Hospital, Beijing, China, 4College of Engineering, Peking University, Beijing, China
Synopsis
Reduced plantar sensation can lead to weakened balance ability in elder adults and an addition of cognitive tasks will further weaken it. Thereby, we attempted to explore the brain activity pattern of the elder and of young adults under foot stimuli and in dual-task condition. The results revealed that elder adults have significantly stronger cortical excitability than young do, and that foot stimuli induced stronger cortical excitability compared with dual-task condition. In conclusion, these phenomena may be due to the elder adults’ inadequate central reserve. Besides, added cognitive task can further reduce the brain’s response through diminished sensory input.
Introduction
It is generally accepted
that loss of balance has become a common problem in elder adults and the risk
of death or serious injury caused by a fall increases with age[1]. Human balance control is a complex
physiological process and the central nervous system(CNS) plays an important
role in balance control[2]. In addition, as a dynamic equilibrium process, balance
control not only requires maintaining balance but also often necessitates
simultaneous cognitive tasks[3, 4]. And the probability of
occurrence of falls in elder adults with cognitive impairment is twice than that
of normal elder adults[5]. However, central nervous
regulatory mechanisms of weakened balance control under dual-task or multi-task
in daily life are not clear now.Purpose
To better understand the
mechanism of weakened
balance ability in elder adults, we attempted to explore the brain
activity pattern of the elder and of young adults under foot stimuli and dual-task
(cognitive task and foot stimuli) condition by using a self-designed magnetic
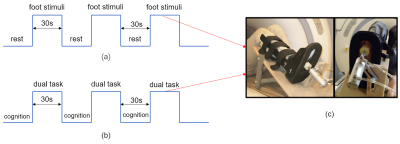
resonance imaging-compatible tactile stimulation system.Materials and Methods
Subjects:
16 healthy young adults (22.2 ± 2.1 years; 11 males) and sixteen healthy elder adults (61.8 ± 6.5 years; 9 males) were recruited for this study. All subjects provided informed consent as approved by the Institutional Review Board of Peking University First Hospital, Beijing.
Protocol:
In the fMRI protocol, each block was 30s in duration and repeated three times(Fig.1). The first part of the fMRI protocol was used to compare the brain response patterns of elder and young adults under the foot stimuli and the second was used to further understand the response of dual-task in elder adults.
MRI images acquisition
MRI scans were performed on a GE 3T scanner (Signa Excite HD). BOLD data were acquired using a standard echo-planar imaging sequence (repetition time/echo time, 2000/30 ms; flip angle, 90°; image matrix, 64×64; thickness/spacing, 4 mm/1 mm; field of view, 230×230 mm2; 28 interleaved axial slices; and 30 repetition times.) A high-resolution structural image was acquired using a three-dimensional fast spoiled gradient echo sequence for anatomical localization (repetition time/echo time, 7.8/3.0 ms; flip angles, 20°; inversion time, 450 ms; field of view, 240×240 mm2; slice thickness, 2.0 mm with 1.0 mm overlap; in-plane resolution, 1×1 mm2.)
Data and statistical analysis
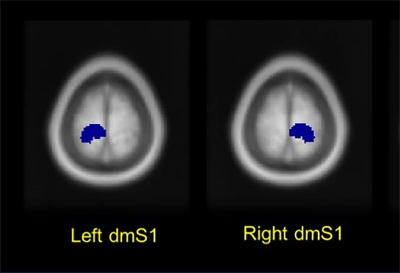
BOLD data were preprocessed with Statistical Parametric Mapping software (SPM8). Next, one sample t-test was used to obtain group analysis results. In addition, two sample t-tests were used to explore difference of brain activity patterns between conditions. Brain regions related to sensory processing of the foot and lower limbs are defined as regions of interest (ROIs), which include the bilateral dorsomedial somatosensory cortex (dmS1) (Fig.2). And the average percent signal change (PSC) value of each ROI was calculated. Then, the differences of PSC values within each ROI were analyzed through two-tailed paired t-tests.
Results
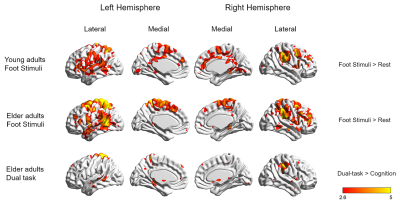
Two sample t-test results showed that compared to young adults, foot stimuli to elder adults elicits increased excitability of bilateral postcentral gyrus, precentral gyrus, supplementary motor area, middle frontal gyrus, inferior parietal lobule, superior parietal lobule, middle cingulate gyrus, angular gyrus, supramarginal gyrus, superior temporal gyrus, middle temporal gyrus, left precuneus, right superior frontal gyrus, and insula (uncorrected, p < 0.001, cluster size ≥ 10).
Whole-brain group-level comparison revealed that, in comparison dual-task condition foot stimuli only was associated with greater activation within the bilateral supplementary motor area, superior frontal gyrus, left side of central lobule, angular gyrus, middle frontal gyrus, right postcentral gyrus, superior parietal gyrus, and supramarginal gyrus in elder adults (uncorrected, p < 0.001, cluster size ≥ 10).
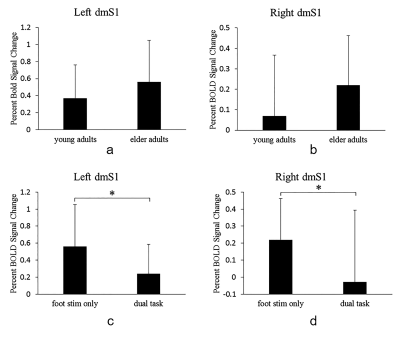
ROI analysis further showed that there was no significant difference in the excitability of the left dmS1 and the right dmS1 between young and elder adults (Fig.4). ROI analysis also revealed that, as compared to foot stimuli only condition, the left dmS1 (p < 0.05) as well as the right dmS1 (p < 0.05) were significantly inhibited under the dual-task condition in elder adults (Fig.4.).
Discussion
This study found that elder adults showed a stronger
cortical response to foot stimuli, and this is probably due to their inadequate
central reserve and lower brain network efficiency. However, there was
significant reduced PSC value in the bilateral dmS1 under dual-task, which
suggested a decrease of sensory input of foot in elder adults. Thus, we believe
that diminished sensory input is likely to be related to weakened balance
control under dual-task or multi-task in the elder adults’ daily lives. Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (grant no. 11372013 and 11572003).References
1.Manchester, D., et al.,Journals of Gerontology, 1989. 44(4): p. M118-M127.
2.Gribble, P.A., et al., Archives of Physical Medicine and Rehabilitation, 2004. 85(4): p. 589-592.
3.Yogev-Seligmann, G., et al., Movement Disorders, 2008. 23(3): p. 329-342.
4.Logsdon, R.G., et al., Alzheimers care today, 2007. 8(4): p. 309-318.
5.Tinetti, M.E., , et al.,New England Journal of Medicine, 1988. 319(26): p. 1701-1707.
Figures