2386
More than BOLD: dual spin populations create functional contrast1Neuroscience, Baylor College of Medicine, Houston, TX, United States, 2Electrical Engineering, University of Texas at Austin, Austin, TX, United States
Synopsis
The "classical" description of functional contrast postulates a single spin population with transverse lifetime modulated by neurovascular coupling. A variety of studies have cast doubt on this description. To better understand such issues, novel methods were used to probe functional contrast in the gray matter of human visual cortex as a function of echo time and flip angle. We find evidence that two spin populations with disparate lifetimes contribute to functional contrast.
Purpose
The "classical" description of functional contrast postulates a single spin population with transverse lifetime modulated by neurovascular coupling. Several studies have cast doubt on this description, showing unexpected dependence on excitation flip angle.1,2 To better understand such issues, novel methods were used to probe functional contrast in the gray matter of human visual cortex as a function of echo time and flip angle.Methods
Functional data were collected using a dual-echo spiral-out sequence3 with 1.5-mm cubic voxels and Tacq = 25 ms. 36 short (108 s) runs were collected, with TE varying from 7—90 ms for the first echo, thus providing 12 TE samples on 7—115 ms. To characterize noise, each TE pair was repeated 6 times. During each run, subjects fixated upon a small (0.1°) with a color that changed every 0.5 s. Subject's task was to press a button every time a specified fixation-dot color was detected. Surrounding fixation, a full-field (1—9°) high-contrast flickering-dot stimulus was displayed for 12 s interleaved with 12-s blank periods. This cycle was repeated 3.5 times per run; the first 12 s of data were discarded to reduce transients. After standard pre-processing, data were transformed into a high-resolution (0.7-mm voxel) reference anatomy that had been segmented to delineate gray matter. Functional data were then averaged across the central 0.2—0.8 of the gray matter using a morphing-based scheme4 to minimize partial volume effects. Data were then transformed onto the gray-white surface, and evaluated in a region-of-interest that was the combination of visual areas V1—3, defined using a population receptive field mapping method5. To remove weak outliers, only strongly responding vertices (p < 0.001) were retained in the ROI; this typically included 80—90% of the whole ROI. For each surface vertex, the functional time series was then fit with a sinusoid to quantify contrast.
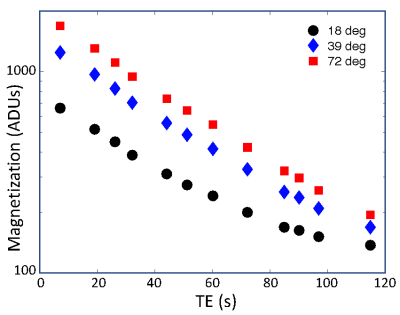
Sessions were conducted for a single 72° flip angle in all subjects to obtain the TE dependence of magnetization signal and contrast. In two flip-angle comparison sessions, runs were performed at three angles 18°, 39°, and 72°, with only two repeats per TE pair.
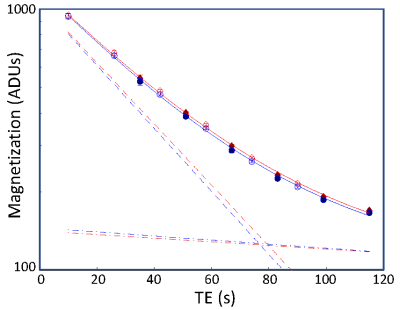
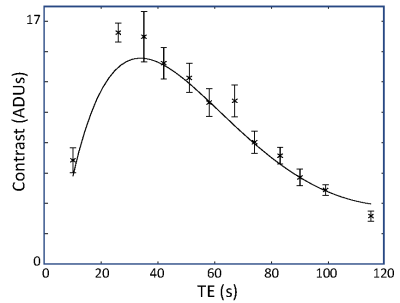
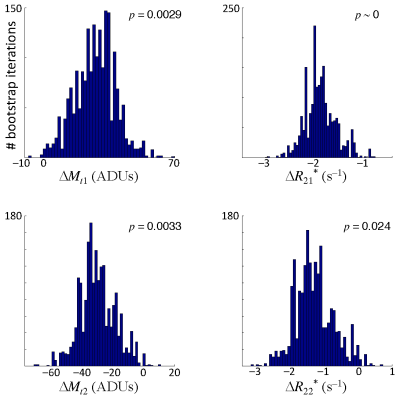
Signal decays were fit with a biexponential function, $$$M_t = M_{1t}\exp\left(-T_{E}R_{21}^*\right)+M_{2t}\exp\left(-T_{E}R_{22}^*\right)$$$. Fits were performed using a gridded search procedure over a reasonable range of parameters for both the rest and active states. We then fixed the mean values for the $$$M_{it}$$$ and $$$R_{2i}^*$$$ parameters, and searched, again using a gridded approach, for the parameter changes between rest and activation, $$$\Delta M_{ti}=M_{ti,active}-M_{ti,rest}$$$ and $$$\Delta R_{2i}^*=R_{2i,active}^{*}-R_{2i,rest}^{*}$$$, that yielded the best fits to the TE dependence of the measured contrast. To obtain confidence intervals, we used bootstrapping across the runs to estimate the distributions all four delta parameters.
Results
Flip-angle comparison sessions show that the echo-time dependence of the signal is not mono-exponential (Fig. 1).The lack of log-linearity is clearest at the two smaller flip angles. In a full session at 72° flip angle, fits to the rest- and active-state data using biexponential fits (Fig. 2) are excellent ($$$R^2 > 99.9$$$%). When the delta parameters are adjusted to fit the contrast data, we obtain good quality fits ($$$R^2 = 95$$$%) to the measured TE dependence of contrast (Fig. 3). When examined for an example subject, all four delta parameters are significant (Fig. 4). The usual BOLD contrast parameter $$$\Delta R_{21}^*$$$ is positive as expected. $$$\Delta M_{t2}$$$ is also positive, while $$$\Delta M_{t1}$$$ is negative. The relaxation rate for the long-lived species, $$$\Delta R_{22}^*$$$, is positive. When analyzed across subjects, $$$\Delta R_{21}^*$$$, $$$\Delta M_{t1}$$$, and $$$\Delta R_{21}^*$$$, are all significant at $$$p < 0.05$$$, while $$$\Delta R_{22}^*$$$ shows a positive trend.Discussion
The results suggest that two spin populations are involved in the generation of functional contrast in gray matter. One population has a relatively short transverse lifetime (~29 ms) but relatively large transverse magnetization, while the other has a longer lifetime (~200 ms) but exhibits much weaker magnetization. One possible interpretation is that the former population corresponds to intravascular and peri-vascular water, while the latter corresponds to extravascular water far enough from all vasculature to be relatively unaffected by hemoglobin susceptibility. The "classic" BOLD response is observed in the first spin pool. The changes in fitted magnetization signal between rest and active state can be understood as an exchange of volume during activation: arterial blood volume increases, forcing a decrease in the long-lived extravascular volume. The participation of these dual spin populations in functional activation helps to explain the observations of "non-classical" variability with flip angle1,2, and enrich our understanding of functional contrast to include the interplay of opposing volume effects.Acknowledgements
Work was supported by NIH R01 NS095933, K25 HL131997, and NSF BCS 1063774.References
1. Renvall, V., Nangini, C., Hari, R., 2014. All that glitters is not BOLD: inconsistencies in functional MRI. Sci Rep 4, 3920.
2. Gonzalez-Castillo, J., Roopchansingh, V., Bandettini, P.A., Bodurka, J., 2011. Physiological noise effects on the flip angle selection in BOLD fMRI. Neuroimage 54, 2764-2778.
3. Singh, V., Pfeuffer, J., Zhao, T., Ress, D., 2017. Evaluation of spiral acquisition variants for functional imaging of human superior colliculus at 3T field strength. Magn Reson Med. doi:10.1002/mrm.26845.
4. Kim, J.H., Ress, D., 2017. Reliability of the depth-dependent high-resolution BOLD hemodynamic response in human visual cortex and vicinity. Magn Reson Imaging 39, 53-63.
5. Greene, C.A., Dumoulin, S.O., Harvey, B.M., Ress, D., 2014. Measurement of population receptive fields in human early visual cortex using back-projection tomography. J Vis 14.
Figures