2383
Sensitivity of passband bSSFP fMRI at 14 Tesla1Centre d'Imagerie Biomédicale, Ecole Polytechnique Fédérale de Lausanne, Lausanne, Switzerland
Synopsis
Passband bSSFP is an excellent alternative to gradient-echo EPI for BOLD fMRI at high field but properties of the BOLD signal have not been reported at ultra-high field (14T). Here, we show that the BOLD amplitude is similar for short and intermediate TR (6 and 12 ms, respectively) which suggests that, in spite of the high field, BOLD contrast in passband bSSFP has limited T2* and off-band contributions, and dominant T2 contributions for TR ≤ 12 ms. A short TR can thus be used to increase temporal or spatial resolution, as well as coverage, with no penalty in intrinsic sensitivity.
Introduction
Passband balanced SSFP (bSSFP) is an excellent alternative to gradient-echo EPI for BOLD fMRI at high field and has been explored in several studies1-5. Simulations predict improved selectivity of capillary-sized vessels at shorter repetitions times (TR) and higher field strengths6-8, hence a strong incentive to use bSSFP fMRI at 14T, with very short timings. However, at ultra-high field, T2*- weighting and off-resonance effects could dominate the passband bSSFP BOLD contrast in spite of the short TR, while the latter is expected to also produce a more limited BOLD contrast7-9, hence potentially forfeiting the benefits. Here we investigate experimentally the TR-dependence of the BOLD signal in passband bSSFP fMRI at 14T.Methods
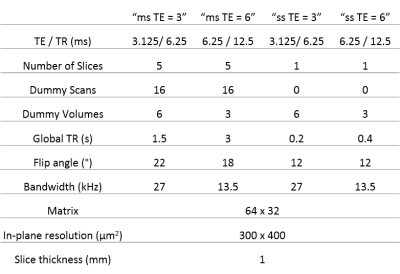
All experiments were approved by the local Service for Veterinary Affairs and performed on a 14T Varian system using a quadrature surface transceiver. Ten Sprague-Dawley rats underwent an fMRI scan under medetomidine sedation (0.1 mg/kg bolus and 0.1 mg/kg/h perfusion). For task-fMRI, a pair of electrodes was inserted in each forepaw. The stimulation paradigm was 21s OFF – 21s ON, repeated for 5’, with 5’ rest between runs. Multi-slice implementations of bSSFP in the transient state have been reported previously10,11 and are also used here. Two sets of TE/TR were compared for single slice acquisitions (true steady-state) and multi-slice (pseudo steady-state) (all parameters in Fig.1). Single- and multi-slice were also compared to each other for the short TE/TR. For each paired comparison, data was acquired on the same paw for two consecutive runs (in random order), before switching to the other paw, etc. In total, 14 pairs of runs were acquired to compare single- and multi-slice bSSFP fMRI, 14 pairs for single-slice TE/TR = 3/6 vs TE/TR = 6/12 ms, and 27 pairs for multi-slice TE/TR = 3/6 vs TE/TR = 6/12 ms.
The data were processed in SPM12 using familywise error correction and p < 0.05. Maximum t-score, cluster volume and BOLD amplitude (averaged over the four voxels with highest activation) were extracted for each cluster. Paired t-tests were run at the two-sided 5% significance level to determine significantly different metrics between protocols.
Results
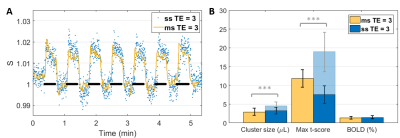
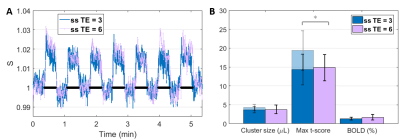
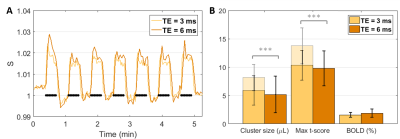
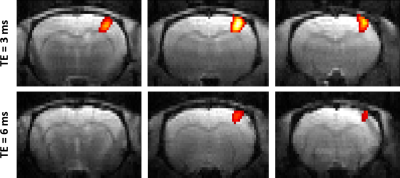
At same TE = 3 ms, both cluster size (p = 4.27E-04) and t-score (p = 8.91E-05) were higher with the single-slice sequence vs multi-slice. The difference was fully accounted by the 7.5 times higher sampling rate of the single-slice acquisition: after temporal down-sampling, cluster sizes were similar while the t-score became significantly lower (p = 1.35E-04) for the single slice protocol due to lower SNR. The BOLD amplitude was comparable between single-slice and multi-slice protocols (Fig. 2). Varying TE (and TR) produced no difference in BOLD amplitude for neither the single-slice (Fig. 3) nor multi-slice protocol (Fig. 4). As such, the short TE acquisition yielded improved sensitivity in terms of activated cluster size and t-score (Fig. 5). However, significant differences in fMRI metrics vanished when temporal resolutions were matched (Figs. 3-4).Discussion and Conclusions
Our results show that, experimentally, at 14T there was no significant difference in BOLD amplitude between TE/TR = 3/6 and TE/TR = 6/12 ms, in the rat cortex where T2 was estimated at 24.9 ± 0.1 ms (data not shown). This suggests that, even at such high field strength, the BOLD contrast in passband bSSFP has limited T2* and off-band contributions, and rather T2 contributions. The benefit of longer TE (and TR) for increased BOLD contrast could be compensated at shorter TE by increased direct contribution from the venous blood (T2 ≈ 9 ms at 9.4T12) and by increased sensitivity (in the extravascular space) to even smaller vessels. These aspects could be corroborated by dedicated simulations which are the object of future work.
The results also support the use of short TR’s for BOLD fMRI with bSSFP: there is no penalty in terms of BOLD sensitivity while the acquisition time is reduced and can be exploited either for increased temporal resolution, or for extended slice coverage.
In parallel, this study further validates the use of multi-slice bSSFP slightly outside the steady-state regime (a few dummy pulses are still applied prior to each slice acquisition). The multi-slice protocol is indeed shown to have similar properties to single-slice for BOLD fMRI, while benefitting from increased SNR and spatial coverage. This implementation could be particularly beneficial in the context of limited acceleration options for 3D bSSFP on animal imaging systems.
Acknowledgements
The authors thank Mario Lepore and Stefan Mitrea for technical assistance with animal setup and monitoring. This work was supported by the Centre d'Imagerie Bio-Médicale (CIBM) of the University of Lausanne (UNIL), the Swiss Federal Institute of Technology Lausanne (EPFL), the University of Geneva (UniGe), the Centre Hospitalier Universitaire Vaudois (CHUV), the Hôpitaux Universitaires de Genève (HUG) and the Leenaards and the Jeantet Foundations.References
1. Zhong, K., Leupold, J., Hennig, J., Speck, O., 2007. Systematic investigation of balanced steady-state free precession for functional MRI in the human visual cortex at 3 Tesla. Magn Reson Med 57, 67-73.
2. Lee JH, Dumoulin SO, Saritas EU et al., 2008. Full-brain coverage and high-resolution imaging capabilities of passband b-SSFP fMRI at 3T. Magn Reson Med 49, 1099-110.
3. Park, S.H., Kim, T., Wang, P., Kim, S.G., 2011. Sensitivity and specificity of high-resolution balanced steady-state free precession fMRI at high field of 9.4T. Neuroimage 58, 168-176.
4. Muir, E.R., Duong, T.Q., 2011. Layer-specific functional and anatomical MRI of the retina with passband balanced SSFP. Magn Reson Med 66, 1416-1421.
5. Miller, K.L., 2012. FMRI using balanced steady-state free precession (SSFP). Neuroimage 62, 713-719.
6. Miller, K.L., Smith, S.M., Jezzard, P., Wiggins, G.C., Wiggins, C.J., 2007. Signal and noise characteristics of SSFP FMRI: a comparison with GRE at multiple field strengths. Neuroimage 37, 1227-1236.
7. Kim, T.S., Lee, J., Lee, J.H., Glover, G.H., Pauly, J.M., 2012. Analysis of the BOLD Characteristics in Pass-Band bSSFP fMRI. Int J Imaging Syst Technol 22, 23-32.
8. Baez-Yanez, M.G., Ehses, P., Mirkes, C., Tsai, P.S., Kleinfeld, D., Scheffler, K., 2017. The impact of vessel size, orientation and intravascular contribution on the neurovascular fingerprint of BOLD bSSFP fMRI. Neuroimage 163, 13-23.
9. Miller, K.L., Jezzard, P., 2008. Modeling SSFP functional MRI contrast in the brain. Magn Reson Med 60, 661-673.
10. Jelescu, I.O., Reynaud, O., da Silva, A.R., Gruetter, R., 2017. Multi-slice balanced SSFP fMRI is an excellent alternative to GE-EPI in the rodent at ultra-high field. Proc. ISMRM 2017, p. 5238.
11. Blazquez Freches, G., Chavarrias, C., Shemesh, N., 2017. Tonotopic mapping in the in vivo mouse via high resolution fMRI. Proc. ISMRM 2017, p. 160.
12. Lee, S.P., Silva, A.C., Ugurbil, K., Kim, S.G., 1999. Diffusion-weighted Spin-Echo fMRI at 9.4 T: Microvascular/Tissue Contribution to BOLD Signal Changes. Magn Reson Med 42, 919-928.
Figures