2376
Perspective taking modulates inter-subject correlated hemodynamic brain responses in movie watching1National Taiwan University, Taipei, Taiwan
Synopsis
We used function Magnetic Resonance Imaging (fMRI) to obtain the hemodynamic brain responses during perspective modulated naturalistic movie presentations to find the influence of perception to individual’s cognitive and affective reactions. Inter-subject correlation (ISC) was used as the analysis tool and Interpersonal Reactivity Index (IRI) was used as the behavioral measurement tool. The study applied selected group ISC analysis to distinguish neural substrates related to physical, cognitive, and affective perspective-taking using naturalistic perspective modulated movie presentation. The finding helps understanding the neural mechanism of perspective taking and would be a useful for future social cognition studies.
INTRODUCTION
Perspective taking (PT) is a skill of putting oneself into other's viewpoint. It is an important cognitive developmental component under the rubric of Theory of Mind (ToM) 1. The spectrum of perspective-taking included physical (or perceptual) perspective-taking (PP) 2, cognitive (or conceptual) perspective-taking (PT) 3, and emotional (or affective) perspective-taking (EC) 4,5. In this research, We used fMRI to obtain the hemodynamic brain responses during perspective modulated movie presentations. Inter-subject correlation (ISC) was used to analyze the synchronized brain activity during movie watching 6,7. While ISC is primarily applied to identify brain regions that are highly synchronized across subjects, we looked for regions that are not synchronized across all subjects but are highly synchronized across a selected group of participants. Interpersonal Reactivity Index (IRI) 8, a measurement categorizing individual’s reactions to stimuli into four subscales: perspective taking (PT) scale, empathic concern scale (EC), personal distress scale (PD) and fantasy scale (FS), was used as the behavioral measurement to contrast between subject group in the ISC analysis. We hypothesized that different sub-groups suggested by IRI exhibit different ISC. These sub-group specific synchronized brain regions suggest the neural correlates for the IRI categorization.METHODS
Nineteen subjects (9 females; age between 20 to 30) were recruited to the fMRI experiment. Four separate movie clips were presented to the subjects during the fMRI scan. Movies clips consisted of two themes and were taken from two perspectives. Movie themes were “junk food consumption” (F) and “pet abandonment” (P). Two themes with the same story line were separately filmed with the first-person perspective (F1, P1) and third-person perspective (F3, P3). IRI written questionnaires were completed by the subjects right after the fMRI experiment.
The fMRI data were preprocessed by SPM and preprocessing including motion correction, slice timing correction, spatial smoothing, and normalization were performed separately for each subject and each session. The voxel-wise contrast of ISC between first (F1-P1) and third (F3-P3) person perspective was computed by a two-sample t-test on the pairwise correlation values of sessions. Contrast of ISC was also done to the selected groups of high versus low PT, EC, PD and FS scales with the same analysis.
RESULTS
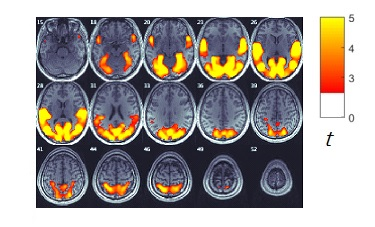
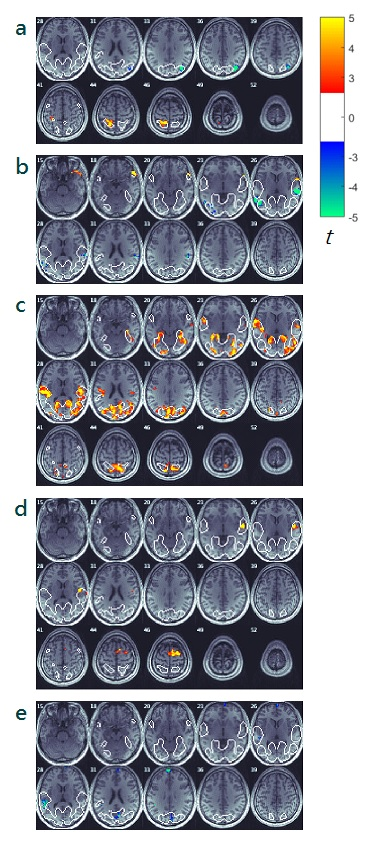
The neural correlation contrast results were overlaid on the result of ISC of the combined BOLD time series [Figure1] to identify the brain regions that are selectively related to PP, PT, EC, PD and FS [Figure2]. PP modulation resulted in significant contrasts in the left superior parietal gyrus junction Precuneus and the right middle occipital gyrus junction angular gyrus. PT modulation resulted in significant contrasts in both sides of superior temporal gyrus. EC modulation resulted in significant contrasts in both sides of Calcarine, Precuneus and cuneus, Rolandic operculum, Heschl’s gyrus. PD modulation resulted in significant contrasts in the right supplementary motor area and superior temporal gyrus.DISCUSSION
The purpose of the present work was to find the influence of perception to an individual’s cognitive and affective reactions. ISC was used to identify neural substrates that are common and distinct between types of stimulus and within chosen groups of subjects. Types of stimulus was modulated by naturalistic movie presentation in first and third person perspective (PP). Subject groupings were divided by their similarity in PT, EC, PD and FS, using IRI. The neural correlation contrast result of physical, cognitive, and affective perspective-taking were found around the temporal-parietal junction (TPJ) but in separable location, which aligns with most of the perspective-taking and empathy studies 2,5,9-14. The neural correlation contrast of PP appeared at regions that previously found to be visual-spatial information processing 15-17, egocentric and allocentric spatial control 18-21, self-reflection and self-judgement 22,23 areas. Further, modulating subject groupings in PT and EC yield a neural correlation contrast around to superior temporal gyrus and superior parietal gyrus and regions of different cognitive and emotional processing areas 5,24. The synchronization found in TPJ and the network of cognitive and emotional processing areas suggest that the perspective-taking spectrum expended, from the visual-spatial processing areas (PP), to TPJ and then spread to temporal cognitive (PT) and emotional (EC) processing areas. To reveal more about the relationships of PP, PT and EC, further studies is required on the connectivity and BOLD activity on these regions.CONCLUSION
The study successfully applied selected group ISC analysis and IRI measurement tool to distinguish neural substrates related to physical, cognitive, and affective perspective-taking using naturalistic movie presentation with the modulation of perspectives. Besides understanding perspective taking more in depth, the neural correlation result would be a useful reference for future researches on cognitive and affective reactions in social cognition studies.Acknowledgements
This work was partially supported by Ministry of Science and Technology, Taiwan (103-2628-B-002-002-MY3, 105-2221-E-002-104), and the Academy of Finland (No. 298131).References
1 Baron-Cohen S., Leslie A. M. & Frith U. Does the autistic child have a “theory of mind”? Cognition.1985; 21:37-46.
2 Filipi A. & Wales R. Perspective-taking and perspective-shifting as socially situated and collaborative actions. Journal of Pragmatics.2004; 36:1851-1884.
3 Barnes-Holmes Y., McHugh L. & Barnes-Holmes D. Perspective-taking and theory of mind: a relational frame account. The Behavior Analyst Today.2004; 5:15.
4 Eisenberg N. & Miller P. A. The relation of empathy to prosocial and related behaviors. Psychological bulletin.1987; 101:91.
5 Hein G. & Singer T. I feel how you feel but not always: the empathic brain and its modulation. Current opinion in neurobiology.2008; 18:153-158.
6 Hasson U., Nir Y., Levy I. et al. Intersubject synchronization of cortical activity during natural vision. science.2004; 303:1634-1640.
7 Pajula J., Kauppi J.-P. & Tohka J. Inter-subject correlation in fMRI: method validation against stimulus-model based analysis. PloS one.2012; 7:e41196.
8 Davis M. H. Measuring individual differences in empathy: Evidence for a multidimensional approach. Journal of personality and social psychology.1983; 44:113-126.
9 Ruby P. & Decety J. Effect of subjective perspective taking during simulation of action: a PET investigation of agency. Nature neuroscience.2001; 4:546-550.
10 Saxe R. & Kanwisher N. People thinking about thinking people: the role of the temporo-parietal junction in “theory of mind”. Neuroimage.2003; 19:1835-1842.
11 Mano Y., Harada T., Sugiura M. et al. Perspective-taking as part of narrative comprehension: a functional MRI study. Neuropsychologia.2009; 47:813-824.
12 Lamm C., Batson C. D. & Decety J. The neural substrate of human empathy: effects of perspective-taking and cognitive appraisal. Journal of cognitive neuroscience.2007; 19:42-58.
13 van der Heiden L., Scherpiet S., Konicar L. et al. Inter-individual differences in successful perspective taking during pain perception mediates emotional responsiveness in self and others: an fMRI study. Neuroimage.2013; 65:387-394.
14 Jackson P. L., Meltzoff A. N. & Decety J. How do we perceive the pain of others? A window into the neural processes involved in empathy. Neuroimage.2005; 24:771-779.
15 Heed T., Beurze S. M., Toni I. et al. Functional rather than effector-specific organization of human posterior parietal cortex. Journal of Neuroscience.2011; 31:3066-3076.
16 Gallivan J. P., McLean D. A., Valyear K. F. et al. Decoding action intentions from preparatory brain activity in human parieto-frontal networks. Journal of Neuroscience.2011; 31:9599-9610. 17 Abdollahi R. O., Jastorff J. & Orban G. A. Common and segregated processing of observed actions in human SPL. Cerebral cortex.2012; 23:2734-2753.
18 Wenderoth N., Debaere F., Sunaert S. et al. The role of anterior cingulate cortex and precuneus in the coordination of motor behaviour. European Journal of Neuroscience.2005; 22:235-246.
19 Hanakawa T., Immisch I., Toma K. et al. Functional properties of brain areas associated with motor execution and imagery. Journal of neurophysiology.2003; 89:989-1002.
20 Adamovich S., August K., Merians A. et al. A virtual reality-based system integrated with fmri to study neural mechanisms of action observation-execution: a proof of concept study. Restorative neurology and neuroscience.2009; 27:209-223.
21 Dohle C., Stephan K. M., Valvoda J. T. et al. Representation of virtual arm movements in precuneus. Experimental brain research.2011; 208:543-555.
22 Kircher T. T., Brammer M., Bullmore E. et al. The neural correlates of intentional and incidental self processing. Neuropsychologia.2002; 40:683-692.
23 Vogeley K., Bussfeld P., Newen A. et al. Mind reading: neural mechanisms of theory of mind and self-perspective. Neuroimage.2001; 14:170-181. 24 Jackson P. L., Brunet E., Meltzoff A. N. et al. Empathy examined through the neural mechanisms involved in imagining how I feel versus how you feel pain. Neuropsychologia.2006; 44:752-761.
25 Gehlbach H. A new perspective on perspective taking: A multidimensional approach to conceptualizing an aptitude. Educational psychology review.2004; 16:207-234.
26 Buckner R. L. & Carroll D. C. Self-projection and the brain. Trends in cognitive sciences.2007; 11:49-57.
Figures