2375
Ventral intermediate nucleus involved in tremor and Postural instability and gait disability-related networks in Parkinson's disease1Department of Radiology, The 2nd Affiliated Hospital, Department of Radiology, School of Medicine, Zhejiang University, hangzhou, China, 2The 2nd Affiliated Hospital, Department of Radiology, School of Medicine, Zhejiang University, hangzhou, China
Synopsis
To investigate the core pathophysiology between Parkinson's disease (PD) motor subtypes in subregions of thalamus and their different directory connectivity patterns, we collected multi-model magnetic resource imaging of 79 PD patients and 31 normal controls. We compared the grey matter volume and perfusion characteristics within the thalamus between PD phenotypes. Granger causality analysis was used to compare the effective connectivity between different subtypes. Our study revealed that core pathophysiology in tremor-dominant subtype may lie in the ventral intermediate nucleus, and a differential effective connectivity pattern existed in tremor and posture instability gait difficulty-related networks that related to behavioral heterogeneity in PD.
Introduction
Parkinson’s disease (PD) patients was categorized into posture instability gait difficulty (PIGD) and tremor-dominant (TD) subtypes according to the predominant motor symptoms for past decades[1]. The disparate neural basis in different subtypes may allow for more personalized treatment. However, the pathophysiological mechanisms of different PD subtypes are not yet well understood. The thalamus is involved in the pathophysiology of both classical striatal-thalamo-cortical (STC)[2] and cerebellothalamic (CTC) circuit[3, 4] in PD. The direction of connectivity differences involving STC and CTC circuit is lacking in PD, which is important for understanding the pathophysiological differences between PD motor subtypes. Therefore, we hypothesize that the subregion of thalamus may be the core pathophysiological region in different PD subtypes, and may mediate different effective connectivity pattern in STC and CTC circuits between PD phenotypes.Methods
79 PD patients (43TD and 36 PIGD) and 31 normal controls (NC) were recruited and scanned using the arterial spin labeling, T1 structural imaging and resting-state functional magnetic resource imaging. We firstly compared the grey matter volume and perfusion characteristics within the thalamus between PD phenotypes by using one-way analysis of variance (P< 0.05, false discovery rate (FDR) corrected), and then located the result subregion within thalamus. Secondly, we performed a 3x2 full ANOVA (full factorial design) with factors Group (TD, PIGD, NC) and Hemisphere (most affected, least affected) to compare the effective connectivity between subregion of thalamus nucleus and the STC and CTC pathways based on Granger causality analysis (GCA, P< 0.05, FDR corrected). Finally, correlation between these variables and motor deterioration were analyzed in PD subtypes.Results
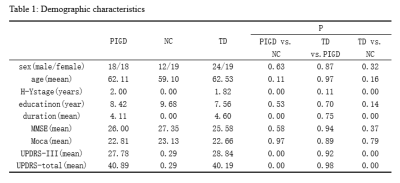
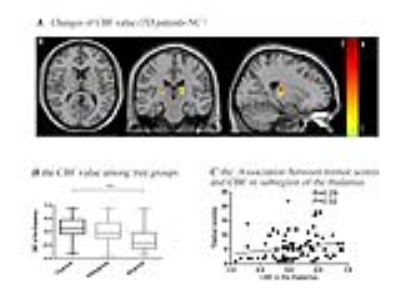
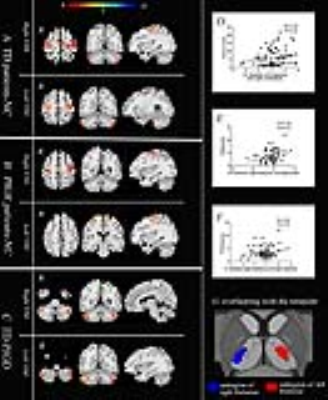
There were no statistically significant differences in gender, age, educational levels or MMSE scores among the three groups (table1). The post hoc analysis exhibited that TD subtype showed significant increased perfusion, but not grey volume, in ventral intermediate nucleus(Vim), where the perfusion value was positively correlated with clinical tremor scores (Fig.1, A-C). Even though the difference of perfusion value between PIGD group and NC didn’t have statistical significance, there was a trend of increased CBF value from NC to TD groups. GCA exposed that TD patients have enhanced effective connectivity from bilateral Vim to contralateral paracentral, bilateral M1 and cerebellum compared with NC. PIGD subtype revealed increased effective connectivity from bilateral Vim to bilateral pre-motor cortex and putamen. Directly comparing TD and PIGD, the connectivity from bilateral Vim to bilateral cerebellum significantly increased in TD. What’s more, there were positive correlations between tremor scores and effective connectivity from Vim to cerebellum. Patients with higher connectivity (from right Vim to right precentral and right putamen) showed higher clinical PIGD scores (Fig. 2).Discussion
This study established that the Vim nucleus perfusion was significantly increased in the TD group, and these perfusion values showed relationship with tremor scores, but this phenomenon was not found in PIGD group. The result highlighted the Vim is the characteristic tremor-related nucleus, which is largely consistent with a recent report[5]. Our findings indicated that the enhanced connectivity from the Vim to the M1 and cerebellum is associated with parkinsonian tremor. This confirmed the Vim-M1-cerebellum circuit is the characteristic pattern optimally described our heterogeneous TD population. The cerebellum is a vital marker in this circuit. It plays a critical role in parkinsonian tremor amplitude and modulation[6]. The putamen is the major input structure of the basal ganglia and receive afferents from thalamus [7]. Single photon emission computed tomography studies found that patients with worse rigidity had more pronounced dopaminergic loss in the posterior putamen[8]. In present study, the Vim nucleus had increased connectivity to putamen in the PIGD patients. What’s more, this connectivity was correlated with the severity of PIGD scores. These findings provide further evidence of a role for the putamen in phenotypic subtype manifestations. We suspected the enhanced connectivity from Vim to the putamen may be a feedback mechanism of dopaminergic loss.Conclusion
This multilevel analysis showed that the core pathophysiology in TD subtype may lie in the Vim, and a differential effective connectivity pattern existed in TD and PIGD-related networks that related to behavioral heterogeneity in PD.Acknowledgements
No acknowledgement found.References
[1] Jankovic J, McDermott M, Carter J, Gauthier S, Goetz C, Golbe L, et al. Variable expression of Parkinson's disease: a base-line analysis of the DATATOP cohort. The Parkinson Study Group. Neurology 1990, 40: 1529-1534. [2] Alves G, Larsen JP, Emre M, Wentzel-Larsen T, Aarsland D. Changes in motor subtype and risk for incident dementia in Parkinson's disease. Mov Disord 2006, 21: 1123-1130. [3] Fukuda M, Barnes A, Simon ES, Holmes A, Dhawan V, Giladi N, et al. Thalamic stimulation for parkinsonian tremor: correlation between regional cerebral blood flow and physiological tremor characteristics. Neuroimage 2004, 21: 608-615. [4] Dirkx MF, den Ouden H, Aarts E, Timmer M, Bloem BR, Toni I, et al. The Cerebral Network of Parkinson's Tremor: An Effective Connectivity fMRI Study. J Neurosci 2016, 36: 5362-5372. [5] Zhang JR, Feng T, Hou YN, Chan P, Wu T. Functional Connectivity of Vim Nucleus in Tremor- and Akinetic-/Rigid-Dominant Parkinson's Disease. CNS Neurosci Ther 2016, 22: 378-386. [6] Helmich RC, Janssen MJ, Oyen WJ, Bloem BR, Toni I. Pallidal dysfunction drives a cerebellothalamic circuit into Parkinson tremor. Ann Neurol 2011, 69: 269-281. [7] Braak H, Bohl JR, Muller CM, Rub U, de Vos RA, Del Tredici K. Stanley Fahn Lecture 2005: The staging procedure for the inclusion body pathology associated with sporadic Parkinson's disease reconsidered. Mov Disord 2006, 21: 2042-2051. [8] Eggers C, Kahraman D, Fink GR, Schmidt M, Timmermann L. Akinetic-rigid and tremor-dominant Parkinson's disease patients show different patterns of FP-CIT single photon emission computed tomography. Mov Disord 2011, 26: 416-423.Figures