2374
Functional connectivity of intrinsic brain networks in chronic low back pain1Arthritis UK Pain Centre, University of Nottingham, Nottingham, United Kingdom, 2Sir Peter Mansfield Imaging Centre, University of Nottingham, Nottingham, United Kingdom, 3NIHR Nottingham BRC, Nottingham, United Kingdom
Synopsis
Understanding pathological changes in intrinsic connectivity networks may advance our knowledge of chronic pain. We performed resting state seed-based functional connectivity analysis of main intrinsic brain networks in 34 chronic low back pain patients and 34 healthy controls. Results of present study are in accordance with studies that demonstrated weaker connectivity within the default mode network and reduced anticorrelation between the default mode and salience networks in chronic pain. In addition, we have identified abnormal sensorimotor network (SMN) connectivity and more profound medial prefrontal – hippocampal connectivity dysfunction in chronic low back pain.
Introduction
Studying intrinsic connectivity networks may advance our understanding of chronic pain1. Some researchers have reported increased connectivity within the default mode network2 (DMN), whereas, others have found reduced connectivity between its anterior and posterior parts3. There is also a growing body of evidence suggesting involvement of salience network4 (SN) and central executive network5 (CEN) in mechanisms of pain chronification. We aimed to investigate functional connectivity of all three networks in chronic low back pain (CLBP).Methods
Data used in the preparation of this work were obtained from the OpenPain Project (OPP) database (https://www.openpain.org). The OPP project (Principal Investigator: A. Vania Apkarian, Ph.D. at Northwestern University) is supported by the National Institute of Neurological Disorders and Stroke (NINDS) and National Institute of Drug Abuse (NIDA). Data consisted of structural and functional images of 34 CLBP patients (19 males, mean age - 49 years, mean pain duration 16 years) and 34 healthy controls (19 males, mean age - 49 years). Image analysis was performed using standard procedures in FSL 5.0.8 (FMRIB software library). All datasets were denoised using ICA-AROMA6. Seed-based connectivity was calculated using 6 regions of interest (ROI’s) each region representing the network of interest: SN - right anterior insula (rAI), CEN - the left dorsolateral prefrontal cortex (lDLPFC) and the DMN - posterior cingulate cortex (PCC). Additional three ROI masks (frontal medial cortex (mPFC), left hippocampus (lHip) and right hippocampus (rHip)), representing anterior and posterior parts of the DMN, were created using the Harvard-Oxford cortical and subcortical atlases. For subject-level image analyses, time series from each of the ROI’s were used as predictors in individual regression models including 2 nuisance covariates (CSF, WM) using FSL FEAT. Group-level analyses were additionally controlled for mean relative motion and statistical analyses were carried out using FLAME 1 (a mixed-effects general linear model within FSL; FWE-corrected Z>2.3, cluster significance p<0.05) comparing connectivity in CLBP patients vs healthy controls. Z-scores from each clusterfor each subject were extracted for two-sample t-test.Results
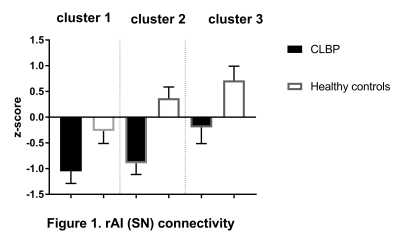
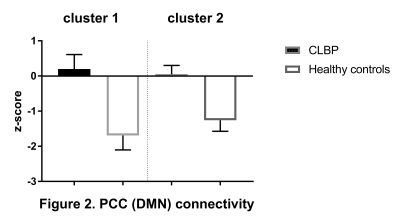
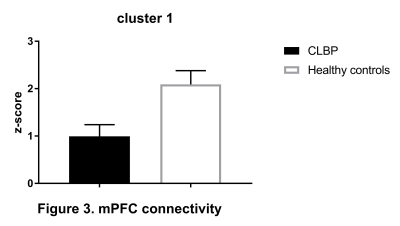
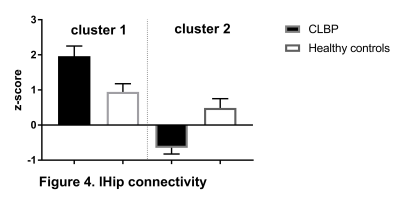
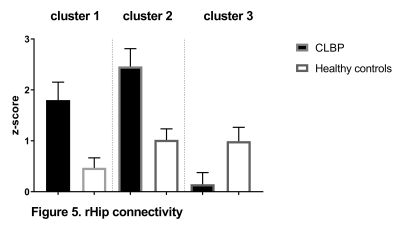
CEN: lDLPFC functional connectivity did not differ between two groups. SN: relative to controls, CLBP patients displayed increased negative (anti-)correlation of rAI with right lateral occipital cortex, occipital pole and fusiform gyrus (Figure 1, cluster 1). Functional connectivity of rAI with right (Figure 1, cluster 2) and left (Figure 1, cluster 3) pre- and postcentral gyri was negative in patients and positive in healthy controls. DMN: CLBP patients demonstrated reduced anticorrelation between PCC and right insula, parietal operculum, angular gyrus, supramarginal gyrus (Figure 2, cluster 2), superior parietal lobule and postcentral gyrus (Figure 2, cluster 1). Functional connectivity between anterior DMN (medial frontal cortex) and posterior cingulate cortex, precuneus, left hippocampus, left parahippocampal gyrus and lingual gyrus was decreased (Figure 3). lHip of CLBP patients showed increased connectivity with right middle and superior temporal gyri (Figure 4, cluster 1) and negative connectivity with anterior cingulate cortex, paracingulate cortex, medial prefrontal cortex and frontal pole (Figure 4, cluster 2). rHip had positive, although decreased connectivity with the same regions (Figure 5, cluster 3) and showed increased connectivity with middle and superior temporal gyri bilaterally (Figure 5, cluster 1,2).Discussion
Our results are in accordance with studies that have demonstrated weaker connectivity within the DMN and reduced anticorrelation between the DMN and SN in chronic pain3,8. In addition, we have identified abnormal sensorimotor network (SMN) connectivity which has not been reported previously3. We found that in CLBP in contrast to pain-free controls SMN is anticorrelated with SN and less anticorrelated with DMN. In comparison with previous research that has reported reduced mPFC-Hip functional connectivity during transition from subacute (pain duration<1 year) to chronic low back pain7, we observed negative mPFC-Hip connectivity in CLBP patients with mean pain duration of 16 years. Additionally, we found increased hippocampal connectivity with middle and superior temporal gyri, which may represent compensatory mechanisms. These abnormal inter-network connectivity relationships may appear due to sustained painful input4,7.Conclusions
We confirm large-scale network disruption of DMN and SN in CLBP and demonstrate for the first time impaired functional connectivity of SMN. Furthermore, observed changes in the hippocampal connectivity may underpin chronification of pain.Acknowledgements
We would like to thank Arthritis Research UK, Haydn Green Foundation and the Vice-Chancellor scholarship program for the financial support and OpenPain Project for providing data for this work.References
1. Apkarian AV, Hashmi JA, Baliki MN. Pain and the brain: specificity and plasticity of the brain in clinical chronic pain. Pain. 2011; 152(3):S49–S64.
2. Kucyi A, Moayedi M, Weissman-Fogel, et al. Enhanced medial prefrontal-default mode network functional connectivity in chronic pain and its association with pain rumination. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2014;34(11):3969-3975.
3. Baliki MN, Mansour AR, Baria AT, et al. Functional reorganization of the default mode network across chronic pain conditions. PloS one. 2014;9(9):e106133
4. Borsook D, Edwards R, Elman I, et al. Pain and analgesia: the value of salience circuits. ProgNeurobiol. 2013; 104:93–105.
5. Apkarian VA, Sosa Y, Krauss BR, et al. Chronic pain patients are impaired on an emotional decision-making task. Pain. 2004; 108(1):129–136.
6. Griffanti L, Salimi-Khorshidi G, Beckmann CF, et al. ICA-based artefact removal and accelerated fMRI acquisition for improved resting state network imaging. NeuroImage. 2014;95:232-247.
7. Mutso AA, Petre B, Huang L, et al. Reorganization of hippocampal functional connectivity with transition to chronic back pain. Journal of Neurophysiology. 2014;111(5):1065-1076.
8. Hemington KS, Wu Q, Kucyi A, et al. Abnormal cross-network functional connectivity in chronic pain and its association with clinical symptoms. Brain structure & function. 2016;221(8):4203-4219.