2371
Effect of emotional enhancement of memory on recollection process in young adults: The influence factors and neural mechanisms1Department of Radiology, The First Affiliated Hospital of Anhui Medical University, Hefei, China, 2GE Healthcare China, Shanghai, China
Synopsis
This research explored how the inherent stimulus properties and amount of devoted attention influenced the emotional enhancement of memory (EEM) effect on recollection and evaluated the correlations between emotional memory/EEM and the spontaneous brain activity of hippocampus, perirhinal, and entorhinal cortex, and the correlations between emotional memory/EEM and the topological properties of three stipulated emotional memory processing networks in 59 young adults using resting-state fMRI. The EEM was elicited by incidental encoding, negative images, and positive high-arousal images. The hippocampus, perirhinal, and entorhinal cortex play distinct roles in the recollection and familiarity processes of emotional memory and the EEM effect.
INTRODUCTION
Emotional experiences can be described by two orthogonal dimensions: arousal (how calming or exciting) and valence (how negative or positive), which dividing emotional stimuli into five categories: negative with high arousal, negative with low arousal, neutral, positive with high arousal, and positive with low arousal. Emotional stimuli are more easily remembered than neutral stimuli, a phenomenon known as the emotional enhancement of memory (EEM).1 According to the dual-process theory, memory recognition relies on both familiarity and recollection. It has been proposed that the EEM effect specifically modulates the former rather than the latter.2 However, the influence factors are not fully understood. Some previous literature have suggested that the hippocampus, perirhinal cortex and entorhinal cortex play distinct roles in recollection and familiarity.3 Nevertheless, how they functioned during those two processes in emotional memory remains unclear. And little is known about the correlations between those critical brain regions and the unique EEM effect of emotional memory. The purposes of present study were to explore the correlations between the hippocampus, perirhinal cortex, entorhinal cortex and emotional memory/EEM effect during the recollection or familiarity process, and to investigate how the EEM effect was influenced by the nature of encoding (incidental or intentional) and the inherent properties (valence and arousal) of emotional stimuli.METHODS
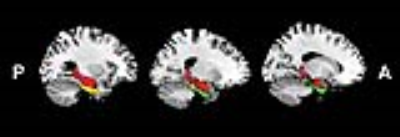
All the subjects gave written informed consent to participate the study, which was approved by the local ethical committee. 59 healthy right-handed young adults participated in emotional picture recognition tests following with the classic remember-know paradigm and MRI scanning. Thirty-five axial slices covering the whole brain were acquired using a 3.0T GE 750w MR scanner (GE Healthcare, Milwaukee, WI) with an 24-channel phase array head coil (TR/TE 2000/30 ms, flip angle 90°, matrix 64 × 64, FOV 220 mm, thickness/gap 3/1mm, total 185 volumes). The amplitude of low-frequency fluctuation (ALFF) and graph theory analysis methods were conducted to find correlations between the hippocampus, perirhinal cortex, entorhinal cortex and recognition sensitivity of emotional memory/EEM effect during recollection (symbolized by Pr) and familiarity process (symbolized by d'). The Human Brainnetome Atlas4 was used to identify brain regions of interest: right and left hippocampus, right and left perirhinal cortex, and right and left entorhinal cortex (Fig. 1). Each pair along with 19 selected brain anatomical coordinates associated with emotion processing and regulation5 constituted the 21 nodes of the hippocampus-, perirhinal cortex-, and entorhinal cortex-mediated emotional memory processing networks. Time courses of each ROI were extracted and correlation matrices were calculated. The two-way analysis of variance (ANOVA) with repeated measures were used for statistical analysis. And the Bonferroni correction was used for post-hoc multiple comparisons to control the false positive rate (corrected p<0.05).RESULTS
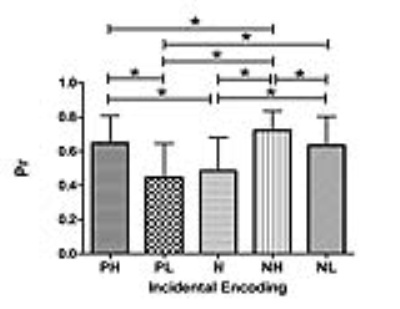
EEM was elicited only by incidental encoding, negative images, and positive high-arousal images (Fig. 2). The emotional memory processing network mediated by the hippocampus had higher clustering coefficient, local efficiency and normalized characteristic path length, but lower normalized global efficiency than those mediated by the perirhinal and entorhinal cortex (Fig. 3). The regional network properties of the right hippocampus were positively correlated with Prnegative-low-arousal, Prpositive-high-arousal, Prpositive-low-arousal, and Prneutral but negatively correlated with EEMnegative-high-arousal. The regional network properties of the entorhinal cortex were positively correlated with Prpositive-low-arousal and Prnegative-low-arousal. The amplitude of low-frequency fluctuations of bilateral entorhinal cortex and left perirhinal cortex were positively correlated with d'negative-low-arousal. The node degree and node efficiency of left entorhinal cortex was negatively correlated with d'positive-high-arousal while the node betweenness of right entorhinal cortex was positively correlated with d'negative-high-arousal.DISCUSSION AND CONCLUSION
The EEM effect do specifically modulated recollection rather than familiarity, but this privilege EEM effect is influenced by the intrinsic properties of emotional stimuli and the involved attention during encoding phase. The hippocampus, perirhinal cortex and entorhinal cortex participate in emotional memory processing but play distinct roles in the recollection and familiarity processes of emotional memory and EEM effect. The hippocampus was particularly associated with recollection sensitivity of emotional memory and the EEMnegative-high-arousal effect. The entorhinal cortex was associated with both recollection and familiarity sensitivity, but showed different correlation patterns. The perirhinal cortex was highly correlated with familiarity sensitivity of negative low-arousal stimuli. Those findings would help to enrich the understanding of the neural mechanisms associated with recollection and familiarity processes of emotional memory and EEM effect.Acknowledgements
No acknowledgement found.References
1. Hamann S. Cognitive and neural mechanisms of emotional memory. Trends Cogn Sci. 2001; 5 (9): 394-400.
2. Dolcos F, LaBar KS, Cabeza R. Remembering one year later: role of the amygdala and the medial temporal lobe memory system in retrieving emotional memories. Proc Natl Acad Sci U S A. 2005; 102 (7): 2626-2631.
3. Montaldi D, Mayes AR. The role of recollection and familiarity in the functional differentiation of the medial temporal lobes. Hippocampus. 2010; 20 (11): 1291-1314.
4. Fan L, Li H, Zhuo J, Zhang Y, Wang J, Chen L, Yang Z, Chu C, Xie S, Laird AR, Fox PT, Eickhoff SB, Yu C, et al. The Human Brainnetome Atlas: A New Brain Atlas Based on Connectional Architecture. Cereb Cortex. 2016; 26 (8): 3508-3526.
5. Cisler JM, James GA, Tripathi S, Mletzko T, Heim C, Hu XP, Mayberg HS, Nemeroff CB, Kilts CD. Differential functional connectivity within an emotion regulation neural network among individuals resilient and susceptible to the depressogenic effects of early life stress. Psychol Med. 2013; 43 (3): 507-518.
Figures