2367
Brain fMRI responses during spinal cord stimulation in rats1Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States, 2Neural Engineering Laboratory, Mayo Clinic, Rochester, MN, United States
Synopsis
Spinal cord stimulation (SCS) has had success in pain management and promising results were demonstrated in other pathologies. To our knowledge, no preclinical studies of SCS in combination with brain fMRI exist, which limit exploration of novel SCS strategies. Here, we show our first results of simultaneous SCS and brain fMRI in rats aiming to establish a framework for future SCS developments. Stimulating spinal cord segment L2 induced a BOLD activation in the primary somatosensory/motor cortex and the thalamus that was dependent on the stimulation frequency. These results demonstrate that monitoring modulation of brain activity due to SCS is feasible.
Introduction
Following the revolutionary expansion of device-based therapies as novel approaches for autonomic neuromodulation to treat conditions such as, e.g., hypertension1, it has been recognized that there is an urgent need for developing neuromodulation strategies using spinal cord stimulation (SCS), median and vagal nerve stimulation and others. SCS is effectively used for pain management2, and first attempts of restoring spinal circuitry function by SCS have already been demonstrated with highly promising results for treatment of motor dysfunction3,4. SCS also has great potential for neuromodulation of peripheral organs such as the kidneys5. Few studies using simultaneous SCS and brain fMRI have been conducted in humans6-9. However, similar studies in animal models have not been reported so far in the literature, thus foreseeably limiting the opportunity of conducting systematic evaluations of novel SCS paradigms. Hence, the goal of this study goal is to establish a framework for spinal cord neuromodulation developments in small animals. fMRI of the brain during SCS may provide insight into the mechanisms of success or failure of SCS treatment, and it could be used to evaluate optimal SCS paradigms.
Here, we show the results of our first attempts at detecting activation in the primary somatosensory/motor cortex (S1/M) and the thalamus in response to different SCS paradigms that employed various stimulation frequencies. Motor cortex and thalamus are expected to exhibit activation during SCS being directly connected to the spinal cord.
Methods
After laminectomy of L2 vertebra, a monopolar epidural Teflon coated platinum wire electrode with an approximately 1 mm long conducting contact was placed on top of the spinal cord in the middle, and secured with sutures to the dura. A subcutaneous Ag/AgCl ground electrode was placed in the back of the rat. Functional brain imaging was conducted on 6 rats at 9.4 T using SE-EPI with the following parameters: TR = 1.5 s, two shots, TE = 35 ms, readout bandwidth = 250 kHz, voxel resolution 0.5 x 0.5 x 1.0 mm3 and 15 slices. Stimulation paradigm consisted of three blocks of 60 s of rest and 18 s of stimulation using 500 µs symmetric biphasic square pulses with amplitude between 0.15 - 0.6 mA. Stimulation amplitudes were set on the bench as to induce visible but minimal body motion. Separate trials were conducted for stimulation frequencies of 5, 20, 40, 80, 160, 320 and 640 Hz in a pseudorandom order. Activation maps were calculated using SPM8 (pFWE < 0.05). Frequency dependence of the BOLD response was assessed using bilateral regions of interest (ROIs) in S1/M and in the thalamus (rostral-caudal from -1.30 till -3.30 mm10).Results
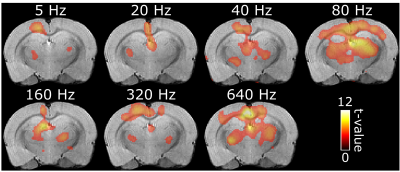
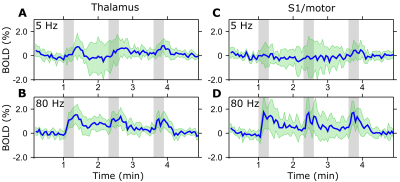
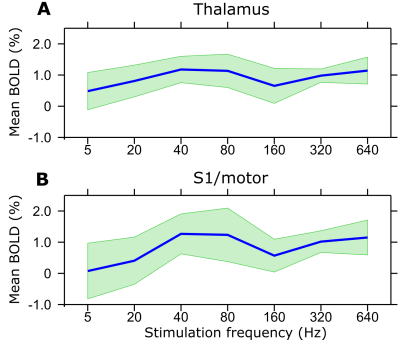
Despite some variability of responses was observed in the animals studied so far, BOLD activation in the brain showed a consistent dependence on the frequency of stimulation of the spinal cord. The activation was mainly localized in the thalamus and S1/M (Figure 1) although also other regions were activated caudally (not shown). Strongest activation for both regions was seen using stimulation frequencies of 40 and 80 Hz while the lowest response was at 5 Hz (Figures 1-3). The response appeared to decrease with lower stimulation frequencies, yet considerable brain activation was still recorded with the highest frequency of 640 Hz.Discussion
A vital component for developing effective and specific spinal cord stimulations is being able to monitor non-invasively the effects of the stimulation on the brain, which is critically important also for understanding circuitry and addressing aspects of neuroplasticity. Here we showed that brain responses can be effectively monitored by fMRI upon epidural stimulation of the spinal cord in a rat model. Brain activation patterns were strongly affected by the frequency of the stimulation, thus demonstrating the sensitivity of the methodology for monitoring the involvement of brain circuitry during different SCS paradigms. Although simultaneous electrophysiology and SCS studies have been conducted in sheep11 and rats12 using intradural and direct current SCS, no prior studies were reported on fMRI detection of brain responses following SCS in rats.Conclusion
With this study we have demonstrated feasibility of brain fMRI during SCS in a rat model, and showed that brain activation patterns strongly depend on the frequency of the SCS. Future work aims at including use of multichannel electrodes which allow orientation selective stimulation13 for disentangling different brain-spinal cord circuitry.Acknowledgements
We gratefully acknowledge our funders: NIH P41 EB015894 and 1U01NS103569-01, WM KECK Foundation, The Emil Aaltonen Foundation (LJL, Finland) and Instrumentarium Science Foundation (HL, Finland).References
1. Victor, R. G. Carotid baroreflex activation therapy for resistant hypertension. Nat Rev Cardiol. 2015;12(8): 451-463.
2. Song, J. J., Popescu, A.andBell, R. L. Present and potential use of spinal cord stimulation to control chronic pain. Pain Physician. 2014;17(3): 235-246.
3. Harkema, S., Gerasimenko, Y., Hodes, J. et al. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet. 2011;377(9781): 1938-1947.
4. Grahn, P. J., Lavrov, I. A., Sayenko, D. G. et al. Enabling Task-Specific Volitional Motor Functions via Spinal Cord Neuromodulation in a Human With Paraplegia. Mayo Clin Proc. 2017;92(4): 544-554.
5. Banek, C. T., Knuepfer, M. M., Foss, J. D. et al. Resting Afferent Renal Nerve Discharge and Renal Inflammation: Elucidating the Role of Afferent and Efferent Renal Nerves in Deoxycorticosterone Acetate Salt Hypertension. Hypertension. 2016;68(6): 1415-1423.
6. Deogaonkar, M., Sharma, M., Oluigbo, C. et al. Spinal Cord Stimulation (SCS) and Functional Magnetic Resonance Imaging (fMRI): Modulation of Cortical Connectivity With Therapeutic SCS. Neuromodulation. 2016;19(2): 142-153.
7. Moens, M., Droogmans, S., Spapen, H. et al. Feasibility of cerebral magnetic resonance imaging in patients with externalised spinal cord stimulator. Clin Neurol Neurosurg. 2012;114(2): 135-141.
8. Moens, M., Sunaert, S., Marien, P. et al. Spinal cord stimulation modulates cerebral function: an fMRI study. Neuroradiology. 2012;54(12): 1399-1407.
9. Stancak, A., Kozak, J., Vrba, I. et al. Functional magnetic resonance imaging of cerebral activation during spinal cord stimulation in failed back surgery syndrome patients. Eur J Pain. 2008;12(2): 137-148.
10. Paxinos, G.andWatson, C. The rat brain atlas in stereotaxic coordinates. (Academic Press, 1998).
11. Flouty, O. E., Oya, H., Kawasaki, H. et al. Intracranial somatosensory responses with direct spinal cord stimulation in anesthetized sheep. PLoS One. 2013;8(2): e56266.
12. Aguilar, J., Pulecchi, F., Dilena, R. et al. Spinal direct current stimulation modulates the activity of gracile nucleus and primary somatosensory cortex in anaesthetized rats. J Physiol. 2011;589(Pt 20): 4981-4996.
13. Lehto, L. J., Slopsema, J. P., Johnson, M. D. et al. Orientation selective deep brain stimulation. J Neural Eng. 2017;14(1): 016016.
Figures