2365
Information content carried by resting-state BOLD fMRI signals reduces differentially in sensory and memory compared with cognitive systems in MCS and UWS patients1Medical College of Wisconsin, Milwaukee, WI, United States, 2Army General Hospital, Beijing, China, 3Beijing Institute of Basic Medical Sciences, Beijing, China
Synopsis
How brain injuries affect the information content carried by signals of brain imaging modalities in patients with consciousness disorders has received little attention. We proposed a novel principal-components-analysis-based approach to quantify regional information content in patients in a minimally conscious state (MCS) and with unresponsive wakefulness syndrome (UWS). We show a reduction of regional information content in both patient populations. Importantly, our analyses revealed differential patterns in the reduction of information content in the sensory and memory compared with high-order cognitive systems in MCS and UWS; such observations are consistent with the clinical symptoms in the two DOC patient populations.
Introduction
Understanding the neuropathological mechanisms underlying severe disorders of consciousness (DOC) in patient populations remains one of the central challenges in clinical neuroscience.1 Information Integration Theory proposes that the level and richness of consciousness depend on the information capacity and the amount of integrated information in the brain.2, 3 Brain injuries, of either traumatic or anoxic nature, may lead to diminished consciousness in patients by reducing the brain’s information capacity or disrupting its ability to integrate information. Despite extensive functional imaging-based network connectivity studies on consciousness disorders,1, 4 how brain injuries affect the information content carried by signals of brain imaging modalities in DOC patients has received little attention. Here we propose a novel approach to quantify changes of regional information content in the brain by assessing the entropy delineated by the principal components (PCs) of regional voxel-based functional imaging signals in clinical patients in a minimally conscious state (MCS) and with unresponsive wakefulness syndrome (UWS). Based on the differences in the clinical symptoms of MCS and UWS,5 we hypothesized (1) that reduced regional information content are present in both MCS and UWS relative to healthy control, and (2) that as the symptoms of DOC deepen from MCS to UWS, UWS exhibits more reduced information content in sensory and high-order cognitive systems of the brain compared with both healthy control and MCS.Methods
Resting-state functional magnetic resonance imaging (rs-fMRI) was performed in 31 UWS patients, 23 MCS patients, and 20 age-matched healthy control individuals. Standard imaging preprocessing procedures were performed. Cleaned voxel BOLD fMRI signals were standardized to z-scores and bandpass filtered within 0.01–0.1 Hz. Regional information content was quantitatively assessed by the entropy (H, Eq. 1) of the PCs of voxel-based BOLD fMRI signals contained within individual neuroanatomical regions,6 $$H=(1/2)*log((2\pi e)^{n}*det(COV))$$ where n is the number of the first few PCs determined by setting a threshold of the total percentage of variance explained (PVE; 85% in this study), COV is the covariance matrix of PVE-determined PCs in a specific region, and det(COV) denotes the determinant of the covariance matrix.
Results
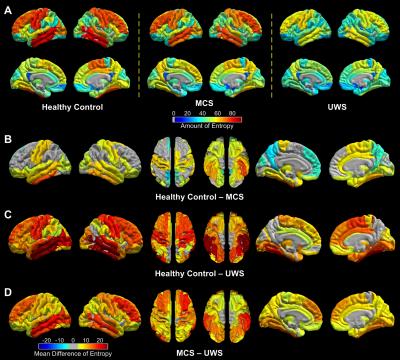
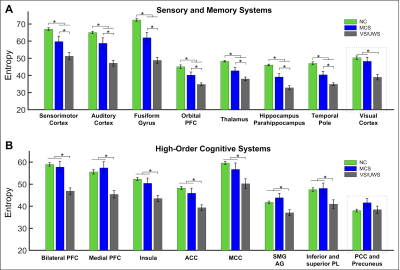
One-sample Kolmogorov-Smirnov tests showed that 99.82%, 99.65%, and 95.05% of all PCs in all brain regions and all subjects in healthy control, MCS, and UWS can be considered coming from a Gaussian distribution, supporting the calculation of entropy using Eq. 1. Regional entropy obtained across the brain in healthy individuals and patients varied in a group-dependent manner with the highest group mean entropy found in healthy control (Fig. 1A left), which was moderately reduced in MCS (Fig. 1A middle) and showed a substantial reduction in UWS (Fig. 1A right). Detailed analyses revealed that, as the symptoms of DOC deepen from MCS to UWS, regional information content also was significantly reduced in that order in the sensory and memory systems of the brain (Fig. 1B–D; Fig. 2A). In contrast, with few exceptions, regional information content in high-order cognitive systems remained statistically at a similar level as those in healthy controls in MCS patients and only showed a significant reduction in UWS patients (Fig. 2B).Discussions and Conclusions
These findings provide, within the current theoretical context of human consciousness, direct evidence that diminished consciousness in MCS and UWS is associated with a reduction of information content in the brain carried by regional BOLD rs-fMRI signals. Further, the findings reveal, for the first time, differential patterns of the reduction of information content in the sensory and memory compared with cognitive systems in MCS and UWS; such observations are consistent with the manifestations of clinical symptoms in the two DOC patient populations. Together, the findings suggest a systems-level mechanism in term of the alteration of regional information content in the brain that may underlie consciousness disorder in MCS and UWS patient.Acknowledgements
The authors thank Ms. Lydia Washechek, BA, for editorial assistance. The authors declare no conflict of interest.References
1. Giacino, J.T., et al., Disorders of consciousness after acquired brain injury: the state of the science. Nat Rev Neurol, 2014. 10(2): p. 99-114.
2. Tononi, G., An information integration theory of consciousness. BMC Neurosci, 2004. 5: p. 42.
3. Tononi, G. and C. Koch, The neural correlates of consciousness: an update. Ann N Y Acad Sci, 2008. 1124: p. 239-61.
4. Boly, M., et al., Brain connectivity in disorders of consciousness. Brain Connect, 2012. 2(1): p. 1-10.
5. Giacino, J.T. and K. Kalmar, Diagnostic and prognostic guidelines for the vegetative and minimally conscious states. Neuropsychol Rehabil, 2005. 15(3-4): p. 166-74.
6. Tzourio-Mazoyer, N., et al., Automated anatomical labeling of activations
in SPM using a macroscopic anatomical parcellation of the MNI MRI
single-subject brain. Neuroimage, 2002. 15(1): p. 273-89.
Figures