2359
Novel functional MRI study reveals cognitive deficits in diabetic peripheral neuropathy1Queensland Brain Institute, The University of Queensland, Brisbane, Australia, 2Agency for Science Technology and Research, Singapore, Singapore, Singapore, 3Saw Swee Hock School of Public Health, National University of Singapore and National University Health System, Singapore, Singapore
Synopsis
Paradigm design in the functional MRI acquisition is of paramount importance to investigate brain activities non-invasively. This is the first time to introduce imagination tasks of walking on floors with different surface stiffness in studying the consequence of a disease associated with diabetic peripheral neuropathy. Our results from the study-specific paradigms show a strong involvement of central nervous system in diabetic peripheral neuropathy subjects as well as cognitive deficits in sensation as caused by the disease.
Introduction
Functional magnetic resonance imaging (fMRI) has emerged as an effective protocol in clinical research to understand brain pathology and characterize neurological diseases 1. Various fMRI paradigm designs enable the mapping of brain functions in different regions 2, but with limited schemes scanning patients engaged in certain motor activities (e.g. walking). However, it has been found that neural activation can be indirectly measured through imagination tasks 3, opening a possibility to observe functional activations of patients with motor difficulties. Here, we introduce two novel fMRI paradigms for studying central nervous system (CNS) deficits in patients suffering from diabetic peripheral neuropathy (DPN) 4, and compare the results to their diabetic non-neuropathic counterparts. An in-house processing procedure is implemented to attain maximal statistic contrasts.Methods
This study included 33 subjects, 14 of whom were diagnosed with DPN. It was approved by the National Healthcare Group Domain Specific Review Board. Informed consents were obtained from all participants prior to the MRI experiments which were performed on a 3T scanner in the Clinical Imaging Research Center (CIRC, Singapore). A three-dimensional high-resolution $$$T_1$$$-weighted dataset ( $$$1\times1\times1$$$mm voxel , the repetition time ($$$T_R$$$) was 2.3s, the echo time ($$$T_E$$$) was 1.9ms, the inversion time (TI) was 0.9s, the flip angle was 9 degrees, the matrix size was $$$256\times256\times192$$$) was acquired for each subject. The fMRI datasets were acquired using an echo planar imaging (EPI) encoding with the resolution of $$$3.4375\times3.4375\times3.4$$$mm3 and a matrix size of $$$64\times64\times42$$$. The $$$T_R$$$ and $$$T_E$$$ were 3s and 0.03s respectively. The acquisition frames were 90 for a foot dorsi-plantar flexion paradigm and 160 for the imagination tasks with more details explained below and in Fig.1. The total acquisition time for the fMRI study was 21 minutes.
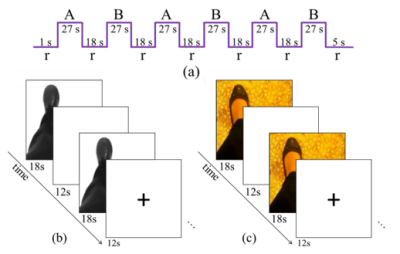
Foot dorsi-plantar flexion paradigm: The subjects engaged in dorsi-plantar flexion exercises in sync with a video. The paradigm used a block design with 18s of rest alternating with 27s of foot movements, with 3 blocks for each foot. This rate is similar to that of foot-flexing movements during walking at casual speeds.
Imagination paradigms: The subjects were allowed to practice walking on both smooth and pebbled surfaces before the scan. They were instructed to take note of the sensation of their feet on the surface, the swing of their legs, and their ease of balance. In the scanner, a clip of a person’s feet walking either on a rough or smooth surface were shown. The subjects were told to imagine walking on the depicted surface with all the accompanying sensations that they previously noted. Two separate tasks (block designs) were used for the smooth and rough surfaces. Each active block lasted for 18s, followed by a resting block of 12s over a total of 16 repetitions. It should be noted that the rough surface shown on the screen was pictured from the floor where the subjects stepped before the scan.
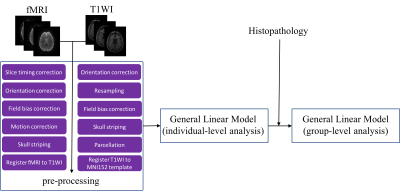
After the acquisition, the dataset of each subject was visually checked to verify that the images were free from major artifacts. Blood-oxygen-level dependent (BOLD) signal processing followed an in-house pipeline as illustrated in Fig.2, which combined the available imaging processing tools to reach the maximal statistic contrasts. A two-sample student t-test was subsequently applied with a significance level of 0.001 and the z threshold of 2.58.
Results
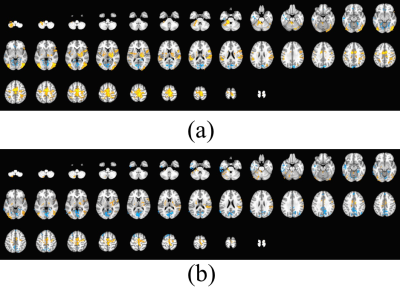
Fig.3 shows the averaged brain's BOLD hemodynamic response to the left dorsiflexion movements in diabetic non-neuropathic and DPN subjects. Both central and peripheral nervous systems were involved. However, the activated regions were not significantly different between groups. The BOLD signal from the right dorsi-plantar flexion task exhibits similar patterns (data not shown).
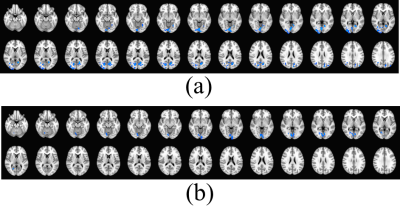
The z-score maps of the differences between the diabetic and DPN groups when performing the imagination tasks are shown in Fig.4. These differences were calculated by subtracting the activities of the diabetic non-neuropathic from the DPN group. Blue stands for negative z values. It is found that subjects without neuropathy showed greater response compared to these with DPN in posterior cingulate and cuneus.
Conclusion and Discussion
This is the first study to map motor imagery deficits in the brain associated with DPN. Our findings demonstrate that pathological changes occur in the CNS along with peripheral nerve damage. The results from the foot dorsiflexion movements failed to show a statistic contrast between DPN and diabetic non-neuropathic subjects, suggesting the need for designing a disease-specific paradigm. The significant differences as revealed by the imagery tasks may indicate that the nature of the DPN disease is not motor related but sensation focused, thus providing an insight into the underlying mechanisms of the DPN disease.Acknowledgements
The authors greatly appreciate funding supports from the Motor Accident and Injury Commission, Australia and the Clinical Imaging Research Center Seed Fund, A*STAR/NUS, Singapore.References
[1] Matthews P M. Clinical Applications of fMRI. In fMRI: From Nuclear Spins to Brain Functions. 2015:611-632. Springer US.
[2] Amaro E and Barker GJ. Study design in fMRI: basic principles. Brain and cognition, 2006;60(3):220-232.
[3] Lotze M, Montoya P, Erb M, et al. Activation of cortical and cerebellar motor areas during executed and imagined hand movements: an fMRI study. Journal of cognitive neuroscience, 1999;11(5):491-501.
[4] Selvarajah D, Wilkinson ID, Maxwell M, et al. Magnetic resonance neuroimaging study of brain structural differences in diabetic peripheral neuropathy. Diabetes Care, 2014;37(6):1681-1688.
Figures