2352
Ketamine-induced modulation of functional connectivity in male and female ratsJaakko Paasonen1, Leena Penna2, Tomi Rantamäki2, and Olli Gröhn1
1A.I.V. Institute for Molecular Sciences, University of Eastern Finland, Kuopio, Finland, 2Department of Biosciences, University of Helsinki, Helsinki, Finland
Synopsis
To get further insights into sustained and gender-dependent neurobiological effects of ketamine, an N-methyl-D-aspartate blocker carrying antidepressant and addictive properties, we investigated the resting-state functional connectivity (FC) in female and male rats 24 hours after a subanesthetic dose of ketamine. Ketamine tended to suppress FC between several brain regions such as hippocampus - medial prefrontal cortex and caudate putamen - medial prefrontal cortex. Significant interactions between treatment and gender were also observed. These observations shed light on the mechanisms underlying the complex neurobiological effects produced by ketamine.
Introduction
Ketamine has been shown to induce a range of long-lasting changes in behavior that cannot be easily explained by its direct pharmacological effects, i.e. the blockade of the glutamatergic N-methyl-D-aspartate receptors. Most notably, a single subanesthetic ketamine treatment produces rapid antidepressant effects in a subset of patients, and the effects sustain long after ketamine has been metabolized 1. Ketamine is differentially metabolized in females and males, which may partially account for the reported gender-dependent behavioral responses to ketamine 2,3. In order to get further insights into the sustained neurobiological effects of ketamine, we investigated the resting-state functional connectivity (FC) in urethane-anesthetized female and male rats 24 hours after a single subanesthetic dose of ketamine that initiates molecular-level signaling events implicated in rapid antidepressant effects.Methods
The animal procedures were approved by the National Animal Experiment Board. Male (n=20) and female (n=20) Wistar rats were used. Ketamine-HCl (50 mg/kg, 2ml/kg, i.p., n=10 in each gender group) or saline (2 ml/kg, n=10 in each gender group) was injected 24 h prior to the functional magnetic resonance imaging (fMRI). For fMRI, rats were anesthetized with urethane (1.25-1.5 g/kg, i.p.), and subsequently fixed to an MRI rat holder with earplugs and bite bar. The rsfMRI data were acquired with single-shot gradient-echo echo-planar imaging sequence with the following parameters: repetition time 1000 ms, echo time 18 ms, 1200 volumes (20 min), field of view 32x32 mm, 15 slices with a thickness of 0.9 mm, matrix size 64x64, and bandwidth 200 kHz. The MRI data were converted to NIfTI (http://aedes.uef.fi), slice-timing corrected, motion-corrected, spatially smoothed, co-registered (SPM8), and band-pass filtered (0.01-0.15Hz). Urethane-induced FC states 4 were separated by observing breathing rate 5, and only data from the rapid-eye-movement (REM)-like state 4 were analyzed (n=5-6 per group). The correlation coefficients between regions of interest were calculated to obtain measures for FC. Connectivity was analyzed from both hemispheres separately to corroborate the findings. Statistical testing was done with two-way ANOVA (uncorrected).Results
The group-level mean FC matrices are
shown in Figure 1, which shows that in both gender groups ketamine-treated rats
tended to have suppressed FC compared to controls. Figure 2 shows the p-value
matrices obtained from two-way ANOVA analyses, indicating that gender alone was
not affecting FC. However, there were several treatment-induced effects on
connectivity of medial prefrontal cortex, caudate putamen, and hippocampus in
both gender groups. Additionally, significant interactions between treatment
and gender were observed, suggesting also different ketamine-induced modulation
of FC between gender groups. Figure 3 shows group-level connectivity values
obtained from representative brain regions associated with depressive disordersDiscussion and conclusions
Wide range of recent functional neuroimaging studies indicate that the FC of several brain regions, such as prefrontal cortex, striatum, and hippocampus, is significantly modulated in depressive disorders 6,7. Prefrontal cortex and hippocampus are key nodes in the default mode network (DMN), where hyperconnectivity is present in depressive disorders. In present study, we report ketamine-induced modulation of FC in rats 24 h after the treatment; decreased connectivity was observed in key regions associated with depressive disorders . Importantly, we observed ketamine-induced suppression of FC within the nodes of the DMN, which may be associated with the antidepressant effects of ketamine. In addition to similar ketamine-induced effects in both gender groups, we also observed interaction between gender and treatment in striatal connections, which can shed light on the mechanisms underlying the gender-dependent effects.Acknowledgements
This work was supported by the Academy of Finland. In addition, we thank Maarit Pulkkinen for technical assistance with the animal preparation.References
1. Berman RM, Cappiello A, Anand A, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47(4):351-354.2. Saland SK, Duclot F, Kabbaj M. Integrative analysis of sex differences in the rapid antidepressant effects of ketamine in preclinical models for individualized clinical outcomes. Curr Opin Behav Sci. 2017;14:19-26.
3. Zanos P, Moaddel R, Morris PJ, et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533(7604):481-486.
4. Zhurakovskaya E, Paasonen J, Shatillo A, et al. Global functional connectivity differences between sleep-like states in urethane anesthetized rats measured by fMRI. PLoS One. 2016;11(5):e0155343.
5. Wilson DA, Hoptman MJ, Gerum SV, Guilfoyle DN. State-dependent functional connectivity of rat olfactory system assessed by fMRI. Neurosci Lett. 2011;497(2):69-73.
6. Brakowski J, Spinelli S, Dorig N, et al. Resting state brain network function in major depression - depression symptomatology, antidepressant treatment effects, future research. J Psychiatr Res. 2017;92:147-159.
7. Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA. Large-scale network dysfunction in major depressive disorder: A meta-analysis of resting-state functional connectivity. JAMA Psychiatry. 2015;72(6):603-611.
Figures
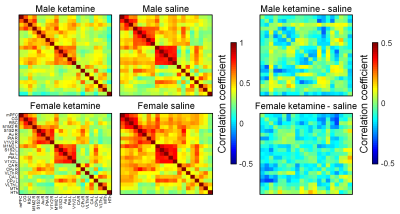
Figure 1. Group-level
correlation matrices and their differences. 21 unilateral regions of
interest were drawn according to an anatomical atlas. In difference matrices,
blue color means decreased correlation value in the ketamine group. Au,
auditory cortex; CA, hippocampus; CG, cingulate cortex; CPu, caudate putamen; HTh,
hypothalamus; M1M2, primary and secondary motor cortex; mPFC, medial prefrontal
cortex; MTh, medial thalamus; PtA, parietal association cortex; RSC,
retrosplenial cortex; S1S2, primary and secondary somatosensory cortex; V1V2,
primary and secondary visual cortex; VLTh, ventrolateral thalamus.
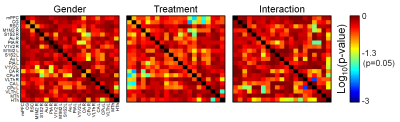
Figure
2. Statistical p-values obtained from two-way ANOVA analysis. Gender
appears not to contribute to the differences in FC between the groups (majority
of p-values >0.05), while treatment affects (p<0.05) FC in several
regions of interest. Additionally, significant interactions (combined effect of
gender and treatment) are observed. For abbreviations, see Figure 1.
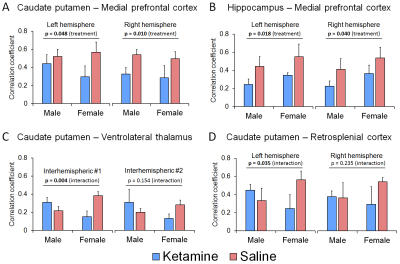
Figure
3. Group-level connectivity values obtained from representative brain regions associated
with the depressive disorder (A-D). A
and B demonstrate connections where ketamine-treatment had similar effect on
both gender groups, while representative connections of differing effects, or
interaction between ketamine and gender, are shown in C and D